Effect of Diet and Exercise on Knee Pain in Patients With Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial
- PMID: 36511925
- PMCID: PMC9856237
- DOI: 10.1001/jama.2022.21893
Effect of Diet and Exercise on Knee Pain in Patients With Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial
Abstract
Importance: Some weight loss and exercise programs that have been successful in academic center-based trials have not been evaluated in community settings.
Objective: To determine whether adaptation of a diet and exercise intervention to community settings resulted in a statistically significant reduction in pain, compared with an attention control group, at 18-month follow-up.
Design, setting, and participants: Assessor-blinded randomized clinical trial conducted in community settings in urban and rural counties in North Carolina. Patients were men and women aged 50 years or older with knee osteoarthritis and overweight or obesity (body mass index ≥27). Enrollment (N = 823) occurred between May 2016 and August 2019, with follow-up ending in April 2021.
Interventions: Patients were randomly assigned to either a diet and exercise intervention (n = 414) or an attention control (n = 409) group for 18 months.
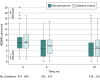
Main outcomes and measures: The primary outcome was the between-group difference in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) knee pain score (range, 0 [none] to 20 [severe]; minimum clinically important difference, 1.6) over 18 months, tested using a repeated-measures mixed linear model with adjustments for covariates. There were 7 secondary outcomes including body weight.
Results: Among the 823 randomized patients (mean age, 64.6 years; 637 [77%] women), 658 (80%) completed the trial. At 18-month follow-up, the adjusted mean WOMAC pain score was 5.0 in the diet and exercise group (n = 329) compared with 5.5 in the attention control group (n = 316) (adjusted difference, -0.6; 95% CI, -1.0 to -0.1; P = .02). Of 7 secondary outcomes, 5 were significantly better in the intervention group compared with control. The mean change in unadjusted 18-month body weight for patients with available data was -7.7 kg (8%) in the diet and exercise group (n = 289) and -1.7 kg (2%) in the attention control group (n = 273) (mean difference, -6.0 kg; 95% CI, -7.3 kg to -4.7 kg). There were 169 serious adverse events; none were definitely related to the study. There were 729 adverse events; 32 (4%) were definitely related to the study, including 10 body injuries (9 in diet and exercise; 1 in attention control), 7 muscle strains (6 in diet and exercise; 1 in attention control), and 6 trip/fall events (all 6 in diet and exercise).
Conclusions and relevance: Among patients with knee osteoarthritis and overweight or obesity, diet and exercise compared with an attention control led to a statistically significant but small difference in knee pain over 18 months. The magnitude of the difference in pain between groups is of uncertain clinical importance.
Trial registration: ClinicalTrials.gov Identifier: NCT02577549.
Conflict of interest statement
Figures

Comment in
-
Diet and Exercise and Knee Pain in Patients With Osteoarthritis and Overweight or Obesity.JAMA. 2023 Apr 18;329(15):1317. doi: 10.1001/jama.2023.2539. JAMA. 2023. PMID: 37071099 No abstract available.
-
Diet and Exercise and Knee Pain in Patients With Osteoarthritis and Overweight or Obesity.JAMA. 2023 Apr 18;329(15):1316-1317. doi: 10.1001/jama.2023.2536. JAMA. 2023. PMID: 37071100 No abstract available.
-
Diet and Exercise and Knee Pain in Patients With Osteoarthritis and Overweight or Obesity.JAMA. 2023 Apr 18;329(15):1317-1318. doi: 10.1001/jama.2023.2533. JAMA. 2023. PMID: 37071101 No abstract available.
References
-
- Vos T, Barber RM, Bell B, et al. ; Global Burden of Disease Study 2013 Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. doi: 10.1016/S0140-6736(15)60692-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

