Long noncoding RNA LEENE promotes angiogenesis and ischemic recovery in diabetes models
- PMID: 36512424
- PMCID: PMC9888385
- DOI: 10.1172/JCI161759
Long noncoding RNA LEENE promotes angiogenesis and ischemic recovery in diabetes models
Abstract
Impaired angiogenesis in diabetes is a key process contributing to ischemic diseases such as peripheral arterial disease. Epigenetic mechanisms, including those mediated by long noncoding RNAs (lncRNAs), are crucial links connecting diabetes and the related chronic tissue ischemia. Here we identify the lncRNA that enhances endothelial nitric oxide synthase (eNOS) expression (LEENE) as a regulator of angiogenesis and ischemic response. LEENE expression was decreased in diabetic conditions in cultured endothelial cells (ECs), mouse hind limb muscles, and human arteries. Inhibition of LEENE in human microvascular ECs reduced their angiogenic capacity with a dysregulated angiogenic gene program. Diabetic mice deficient in Leene demonstrated impaired angiogenesis and perfusion following hind limb ischemia. Importantly, overexpression of human LEENE rescued the impaired ischemic response in Leene-knockout mice at tissue functional and single-cell transcriptomic levels. Mechanistically, LEENE RNA promoted transcription of proangiogenic genes in ECs, such as KDR (encoding VEGFR2) and NOS3 (encoding eNOS), potentially by interacting with LEO1, a key component of the RNA polymerase II-associated factor complex and MYC, a crucial transcription factor for angiogenesis. Taken together, our findings demonstrate an essential role for LEENE in the regulation of angiogenesis and tissue perfusion. Functional enhancement of LEENE to restore angiogenesis for tissue repair and regeneration may represent a potential strategy to tackle ischemic vascular diseases.
Keywords: Angiogenesis; Diabetes; Endothelial cells; Noncoding RNAs; Vascular Biology.
Figures



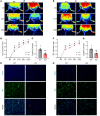
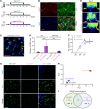
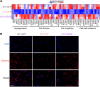



Comment in
-
Epigenetic regulation of angiogenesis and ischemic response by long noncoding RNA LEENE in diabetes.Kidney Int. 2023 Dec;104(6):1048-1050. doi: 10.1016/j.kint.2023.06.025. Epub 2023 Jul 5. Kidney Int. 2023. PMID: 37419449 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA200147/CA/NCI NIH HHS/United States
- R01 GM141096/GM/NIGMS NIH HHS/United States
- R01 HL108735/HL/NHLBI NIH HHS/United States
- R01 HL133254/HL/NHLBI NIH HHS/United States
- UG3 CA256960/CA/NCI NIH HHS/United States
- R01 HL145170/HL/NHLBI NIH HHS/United States
- R01 GM138852/GM/NIGMS NIH HHS/United States
- DP1 DK126138/DK/NIDDK NIH HHS/United States
- R01 DK081705/DK/NIDDK NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- R01 DK065073/DK/NIDDK NIH HHS/United States
- R01 HL106089/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

