Structural Analysis of Phosphorylation Proteoforms in a Dynamic Heterogeneous System Using Flash Oxidation Coupled In-Line with Ion Exchange Chromatography
- PMID: 36512753
- PMCID: PMC9912381
- DOI: 10.1021/acs.analchem.2c04365
Structural Analysis of Phosphorylation Proteoforms in a Dynamic Heterogeneous System Using Flash Oxidation Coupled In-Line with Ion Exchange Chromatography
Abstract
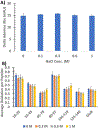
Protein posttranslational modifications (PTMs) are key modulators of protein structure and function that often change in a dynamic fashion in response to cellular stimuli. Dynamic PTMs are very challenging to structurally characterize using modern techniques, including covalent labeling methods, due to the presence of multiple proteoforms and conformers together in solution. We have coupled an ion exchange high-performance liquid chromatography separation with a flash oxidation system [ion exchange chromatography liquid chromatography-flash oxidation (IEX LC-FOX)] to successfully elucidate structural changes among three phosphoproteoforms of ovalbumin (OVA) during dephosphorylation with alkaline phosphatase. Real-time dosimetry indicates no difference in the effective radical dose between peaks or across the peak, demonstrating both the lack of scavenging of the NaCl gradient and the lack of a concentration effect on radical dose between peaks of different intensities. The use of IEX LC-FOX allows us to structurally probe into each phosphoproteoform as it elutes from the column, capturing structural data before the dynamics of the system to reintroduce heterogeneity. We found significant differences in the residue-level oxidation between the hydroxyl radical footprint of nonphosphorylated, monophosphorylated, and diphosphorylated OVA. Not only were our data consistent with the previously reported stabilization of OVA structure by phosphorylation, but local structural changes were also consistent with the measured order of dephosphorylation of Ser344 being removed first. These results demonstrate the utility of IEX LC-FOX for measuring the structural effects of PTMs, even in dynamic systems.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURE
The authors declare the following competing financial interest(s): ZC and JSS disclose a significant interest in GenNext Technologies, Inc., a small company seeking to commercialize technologies for protein higher-order structure, protein-protein interactions, protein-ligand interactions, and PTM analysis.
Figures


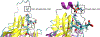

References
-
- Ayoubi TA; Van De Yen WJ Regulation of gene expression by alternative promoters. The FASEB Journal 1996, 10, 453–460. - PubMed
-
- Walsh C Posttranslational modification of proteins: expanding nature's inventory; Roberts and Company Publishers, 2006.
-
- Walsh G; Jefferis R Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 2006, 24, 1241–1252. - PubMed
-
- Walsh CT; Garneau-Tsodikova S; Gatto GJ Jr. Protein posttranslational modifications: the chemistry of proteome diversifications. Angew Chem Int Ed Engl 2005, 44, 7342–7372. - PubMed
-
- Glozak MA; Sengupta N; Zhang X; Seto E Acetylation and deacetylation of non-histone proteins. Gene 2005, 363, 15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

