The BBSome regulates mitochondria dynamics and function
- PMID: 36513220
- PMCID: PMC9792363
- DOI: 10.1016/j.molmet.2022.101654
The BBSome regulates mitochondria dynamics and function
Erratum in
-
Corrigendum to "The BBSome regulates mitochondria dynamics and function molecular metabolism" [Mol Metabol 67 (2023) 101654].Mol Metab. 2024 Dec;90:102038. doi: 10.1016/j.molmet.2024.102038. Epub 2024 Sep 25. Mol Metab. 2024. PMID: 39341604 Free PMC article. No abstract available.
Abstract
Objective: The essential role of mitochondria in regulation of metabolic function and other physiological processes has garnered enormous interest in understanding the mechanisms controlling the function of this organelle. We assessed the role of the BBSome, a protein complex composed of eight Bardet-Biedl syndrome (BBS) proteins, in the control of mitochondria dynamic and function.
Methods: We used a multidisciplinary approach that include CRISPR/Cas9 technology-mediated generation of a stable Bbs1 gene knockout hypothalamic N39 neuronal cell line. We also analyzed the phenotype of BBSome deficient mice in presence or absence of the gene encoding A-kinase anchoring protein 1 (AKAP1).
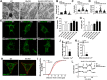
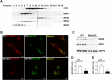
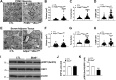
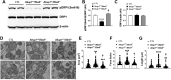
Results: Our data show that the BBSome play an important role in the regulation of mitochondria dynamics and function. Disruption of the BBSome cause mitochondria hyperfusion in cell lines, fibroblasts derived from patients as well as in hypothalamic neurons and brown adipocytes of mice. The morphological changes in mitochondria translate into functional abnormalities as indicated by the reduced oxygen consumption rate and altered mitochondrial distribution and calcium handling. Mechanistically, we demonstrate that the BBSome modulates the activity of dynamin-like protein 1 (DRP1), a key regulator of mitochondrial fission, by regulating its phosphorylation and translocation to the mitochondria. Notably, rescuing the decrease in DRP1 activity through deletion of one copy of the gene encoding AKAP1 was effective to normalize the defects in mitochondrial morphology and activity induced by BBSome deficiency. Importantly, this was associated with improvement in several of the phenotypes caused by loss of the BBSome such as the neuroanatomical abnormalities, metabolic alterations and obesity highlighting the importance of mitochondria defects in the pathophysiology of BBS.
Conclusions: These findings demonstrate a critical role of the BBSome in the modulation of mitochondria function and point to mitochondrial defects as a key disease mechanism in BBS.
Keywords: Bardet-biedl syndrome proteins; Body weight; Leptin sensitivity; Mitochondria.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

