Cardiac DPD-uptake time dependency in ATTR patients verified by quantitative SPECT/CT and semiquantitative planar parameters
- PMID: 36513919
- PMCID: PMC10371940
- DOI: 10.1007/s12350-022-03149-4
Cardiac DPD-uptake time dependency in ATTR patients verified by quantitative SPECT/CT and semiquantitative planar parameters
Abstract
Background: Bone scintigraphy plays an important role in the diagnosis of cardiac Transthyretin-Related Amyloidosis (ATTR). The mechanism of myocardial tracer accumulation and its dependence over time are not fully understood. Recently, a scintigraphic quantification of the cardiac amyloid deposition has been discussed. Nevertheless, little is known regarding the right time of quantitative imaging.
Methods: The geometrical mean of decay corrected total counts over the heart and the heart/whole-body ratio (H/WB) were evaluated in 23 patients undergoing DPD-bone scan with planar whole-body images 1 and 3 hours post injection (p.i.). Myocardial standard uptake values (SUV)peak were assessed in another 15 patients with quantitative SPECT/CT imaging 1 hours and 3 hours p.i..
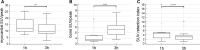
Results: Total counts over the heart (1 hours p.i.: 81,676 cts, range 69,887 to 93,091 cts and 3 hours p.i.: 64,819 cts, range 52,048 to 86,123 cts, P = .0005) and H/WB ratio (1 hours p.i.:0.076 ± 0.020 and 3 hours p.i. 0.070 ± 0.022; P = .0003) were significantly increased 1 hours p.i.. Furthermore median myocardial SUVpeak (1 hours p.i.:12.2, range 9.6 to 18.9 and 3 hours p.i.: 9.6, range 8.2 to 15.0, P = 0.0012) was also significantly higher after 1 hours p.i. compared to 3 hours p.i..
Conclusion: Cardiac DPD activity and myocardial SUVpeak are time-dependent, which should be considered when using quantitative bone scintigraphy in ATTR patients.
Keywords: ATTR; Amyloidosis; SPECT/CT; SUV; quantification; therapy monitoring.
© 2022. The Author(s).
Conflict of interest statement
Diana Bonderman received research grants and honoraria from Pfizer, Alnylam, SOBI and Ionis. Franz Duca received research grants from Pfizer and the Austrian society of cardiology and payment for lectures from Pfizer and Bayer as well as payment for expert testimony from Pfizer and Alnylam. He also received travel support from Pfizer, Novartis, Bayer, AOP and Alnylam. Rene Rettl received speaker fees and congress support from Akcea, Alnylam and Pfizer, as well as well as research grants from Pfizer. The remaining authors declare that they have no competing interests.
Figures
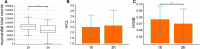
Comment in
-
Time is a gift: time-dependent uptake of DPD in cardiac amyloidosis.J Nucl Cardiol. 2023 Aug;30(4):1372-1373. doi: 10.1007/s12350-023-03244-0. Epub 2023 Apr 25. J Nucl Cardiol. 2023. PMID: 37185771 No abstract available.
References
-
- Lachmann HJ, Hawkins PN. Systemic amyloidosis. Cardiavasc Ren. 2006;6:214–220. - PubMed
-
- Willerson JT, Parkey RW, Bonte FJ, Lewis SE, Corbett J, Buja LM, et al. Pathophysiologic considerations and clinicopathological correlates of technetium-99m stannous pyrophosphate myocardial scintigraphy. Cardiovasc Nucl Med II. 1980;10:54–69. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials

