Plasma markers of COVID-19 severity: a pilot study
- PMID: 36514048
- PMCID: PMC9745704
- DOI: 10.1186/s12931-022-02272-7
Plasma markers of COVID-19 severity: a pilot study
Abstract
Background: SARS-CoV-2 infected patients show heterogeneous clinical presentations ranging from mild symptoms to severe respiratory failure and death. Consequently, various markers reflect this wide spectrum of disease presentations.
Methods: Our pilot cohort included moderate (n = 10) and severe (n = 10) COVID-19 patients, and 10 healthy controls. We determined plasma levels of nine acute phase proteins (APPs) by nephelometry, and full-length (M65), caspase-cleaved (M30) cytokeratin 18, and ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type-1 motif 13) by ELISA. In addition, we examined whole plasma N-glycosylation by capillary gel electrophoresis coupled to laser-induced fluorescence detection (CGE-LIF).
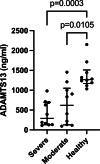
Results: When compared to controls, COVID-19 patients had significantly lower concentrations of ADAMTS13 and albumin (ALB) but higher M30, M65, α1-acid glycoprotein (AGP), α1-antitrypsin (AAT), ceruloplasmin (CP), haptoglobin (HP), and high-sensitivity C-reactive protein (hs-CRP). The concentrations of α1-antichymotrypsin (ACT), α2-macroglobulin (A2MG) and serum amyloid A (SAA) proteins did not differ. We found significantly higher levels of AAT and M65 but lower ALB in severe compared to moderate COVID-19 patients. N-glycan analysis of the serum proteome revealed increased levels of oligomannose- and sialylated di-antennary glycans and decreased non-sialylated di-antennary glycan A2G2 in COVID-19 patients compared to controls.
Conclusions: COVID-19-associated changes in levels and N-glycosylation of specific plasma proteins highlight complexity of inflammatory process and grant further investigations.
Keywords: Acute phase proteins; COVID-9 severity; Cell death; Inflammation; N-glycosylation; Trombosis.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
-
- Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Munoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623–1635. - PubMed
-
- Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

