Antenna-enhanced mid-infrared detection of extracellular vesicles derived from human cancer cell cultures
- PMID: 36514065
- PMCID: PMC9746222
- DOI: 10.1186/s12951-022-01693-2
Antenna-enhanced mid-infrared detection of extracellular vesicles derived from human cancer cell cultures
Abstract
Background: Extracellular Vesicles (EVs) are sub-micrometer lipid-bound particles released by most cell types. They are considered a promising source of cancer biomarkers for liquid biopsy and personalized medicine due to their specific molecular cargo, which provides biochemical information on the state of parent cells. Despite this potential, EVs translation process in the diagnostic practice is still at its birth, and the development of novel medical devices for their detection and characterization is highly required.
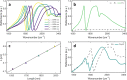
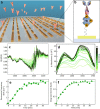
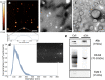
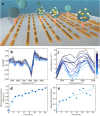
Results: In this study, we demonstrate mid-infrared plasmonic nanoantenna arrays designed to detect, in the liquid and dry phase, the specific vibrational absorption signal of EVs simultaneously with the unspecific refractive index sensing signal. For this purpose, EVs are immobilized on the gold nanoantenna surface by immunocapture, allowing us to select specific EV sub-populations and get rid of contaminants. A wet sample-handling technique relying on hydrophobicity contrast enables effortless reflectance measurements with a Fourier-transform infrared (FTIR) spectro-microscope in the wavelength range between 10 and 3 µm. In a proof-of-principle experiment carried out on EVs released from human colorectal adenocarcinoma (CRC) cells, the protein absorption bands (amide-I and amide-II between 5.9 and 6.4 µm) increase sharply within minutes when the EV solution is introduced in the fluidic chamber, indicating sensitivity to the EV proteins. A refractive index sensing curve is simultaneously provided by our sensor in the form of the redshift of a sharp spectral edge at wavelengths around 5 µm, where no vibrational absorption of organic molecules takes place: this permits to extract of the dynamics of EV capture by antibodies from the overall molecular layer deposition dynamics, which is typically measured by commercial surface plasmon resonance sensors. Additionally, the described metasurface is exploited to compare the spectral response of EVs derived from cancer cells with increasing invasiveness and metastatic potential, suggesting that the average secondary structure content in EVs can be correlated with cell malignancy.
Conclusions: Thanks to the high protein sensitivity and the possibility to work with small sample volumes-two key features for ultrasensitive detection of extracellular vesicles- our lab-on-chip can positively impact the development of novel laboratory medicine methods for the molecular characterization of EVs.
Keywords: Biosensors; Extracellular Vesicles; IR spectroscopy; Nanomaterials; Plasmonics; SEIRA.
© 2022. The Author(s).
Conflict of interest statement
The authors have no competing interests to declare.
Figures

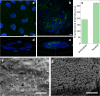
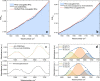
References
-
- Giannopoulou L, Zavridou M, Kasimir-Bauer S, Lianidou ES. Liquid biopsy in ovarian cancer: the potential of circulating miRNAs and exosomes. Transl Res. 2019;205:77–91. - PubMed
-
- Chun-Yan L, Zi-Yi Z, Tian-Lin Y, Yi-Li W, Bao L, Jiao L, et al. Liquid biopsy biomarkers of renal interstitial fibrosis based on urinary exosome. Exp Mol Pathol. 2018;105(2):223–228. - PubMed
-
- Li G, Tang W, Yang F. Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol J. 2020;15(5):1900225. - PubMed
-
- Cui S, Cheng Z, Qin W, Jiang L. Exosomes as a liquid biopsy for lung cancer. Lung Cancer. 2018;116:46–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

