Activation of a Latent Epitope Causing Differential Binding of Antineutrophil Cytoplasmic Antibodies to Proteinase 3
- PMID: 36515151
- PMCID: PMC10191989
- DOI: 10.1002/art.42418
Activation of a Latent Epitope Causing Differential Binding of Antineutrophil Cytoplasmic Antibodies to Proteinase 3
Abstract
Objective: Proteinase 3 (PR3) is the major antigen for antineutrophil cytoplasmic antibodies (ANCAs) in the systemic autoimmune vasculitis, granulomatosis with polyangiitis (GPA). PR3-targeting ANCAs (PR3-ANCAs) recognize different epitopes on PR3. This study was undertaken to study the effect of mutations on PR3 antigenicity.
Methods: The recombinant PR3 variants, iPR3 (clinically used to detect PR3-ANCAs) and iHm5 (containing 3 point mutations in epitopes 1 and 5 generated for epitope mapping studies) immunoassays and serum samples from patients enrolled in ANCA-associated vasculitis (AAV) trials were used to screen for differential PR3-ANCA binding. A patient-derived monoclonal ANCA 518 (moANCA518) that selectively binds to iHm5 within the mutation-free epitope 3 and is distant from the point mutations of iHm5 was used as a gauge for remote epitope activation. Selective binding was determined using inhibition experiments.
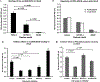
Results: Rather than reduced binding of PR3-ANCAs to iHm5, we found substantially increased binding of the majority of PR3-ANCAs to iHm5 compared to iPR3. This differential binding of PR3-ANCA to iHm5 is similar to the selective moANCA518 binding to iHm5. Binding of iPR3 to monoclonal antibody MCPR3-2 also induced recognition by moANCA518.
Conclusion: The preferential binding of PR3-ANCAs from patients, such as the selective binding of moANCA518 to iHm5, is conferred by increased antigenicity of epitope 3 on iHm5. This can also be induced on iPR3 when captured by monoclonal antibody MCPR2. This previously unrecognized characteristic of PR3-ANCA interactions with its target antigen has implications for studying antibody-mediated autoimmune diseases, understanding variable performance characteristics of immunoassays, and design of potential novel treatment approaches.
© 2022 American College of Rheumatology.
Figures



References
-
- Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheumatol 2013;65:1–11. - PubMed
-
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 1988;318:1651–1657. - PubMed
-
- Jenne DE, Tschopp J, Lüdemann J, Utecht B, Gross WL. Wegener's autoantigen decoded. Nature 1990;346:520. - PubMed
-
- Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies, Arthritis Rheumatol 1998;41:1521–1537. - PubMed
-
- Bossuyt X, Cohen Tervaert JW, Arimura Y, Blockmans D, Flores-Suarez LF, Guillevin L, et al. Position paper: revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683–692. - PubMed

