A remarkable adaptive paradigm of heart performance and protection emerges in response to marked cardiac-specific overexpression of ADCY8
- PMID: 36515265
- PMCID: PMC9822292
- DOI: 10.7554/eLife.80949
A remarkable adaptive paradigm of heart performance and protection emerges in response to marked cardiac-specific overexpression of ADCY8
Abstract
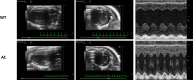
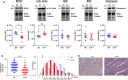
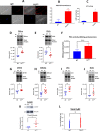
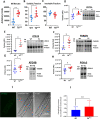
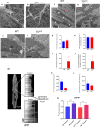
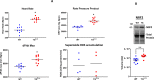
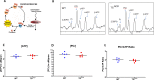
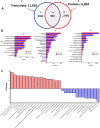
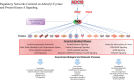
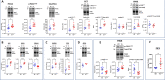
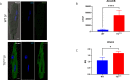
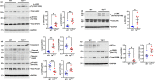
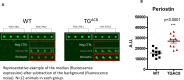
Adult (3 month) mice with cardiac-specific overexpression of adenylyl cyclase (AC) type VIII (TGAC8) adapt to an increased cAMP-induced cardiac workload (~30% increases in heart rate, ejection fraction and cardiac output) for up to a year without signs of heart failure or excessive mortality. Here, we show classical cardiac hypertrophy markers were absent in TGAC8, and that total left ventricular (LV) mass was not increased: a reduced LV cavity volume in TGAC8 was encased by thicker LV walls harboring an increased number of small cardiac myocytes, and a network of small interstitial proliferative non-cardiac myocytes compared to wild type (WT) littermates; Protein synthesis, proteosome activity, and autophagy were enhanced in TGAC8 vs WT, and Nrf-2, Hsp90α, and ACC2 protein levels were increased. Despite increased energy demands in vivo LV ATP and phosphocreatine levels in TGAC8 did not differ from WT. Unbiased omics analyses identified more than 2,000 transcripts and proteins, comprising a broad array of biological processes across multiple cellular compartments, which differed by genotype; compared to WT, in TGAC8 there was a shift from fatty acid oxidation to aerobic glycolysis in the context of increased utilization of the pentose phosphate shunt and nucleotide synthesis. Thus, marked overexpression of AC8 engages complex, coordinate adaptation "circuity" that has evolved in mammalian cells to defend against stress that threatens health or life (elements of which have already been shown to be central to cardiac ischemic pre-conditioning and exercise endurance cardiac conditioning) that may be of biological significance to allow for proper healing in disease states such as infarction or failure of the heart.
Keywords: ROS scavenging; autophagy; cardiac overexpression of human ADCY8; cell biology; left ventricle; mouse; nutrient sensing; proliferation; protection from apoptosis; protein synthesis; proteome; proteosome 35 activity; transcriptome.
Conflict of interest statement
KT, KC, DR, AL, IA, MP, AS, JZ, MW, YL, JQ, MB, MJ, YT, BZ, RT, VK, MR, JL, RB, Rd, SJ, GK, AG, DY, MA, LA, CM, WO, CC, SC, NP, TH, KP, RW, LF, SS, EL No competing interests declared
Figures











References
-
- Adamo L, Staloch LJ, Rocha-Resende C, Matkovich SJ, Jiang W, Bajpai G, Weinheimer CJ, Kovacs A, Schilling JD, Barger PM, Bhattacharya D, Mann DL. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight. 2018;3:e120137. doi: 10.1172/jci.insight.120137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

