Noninvasive Monitoring of Radiation-Induced Salivary Gland Vascular Injury
- PMID: 36515317
- PMCID: PMC10154916
- DOI: 10.1177/00220345221138533
Noninvasive Monitoring of Radiation-Induced Salivary Gland Vascular Injury
Abstract
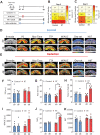
Xerostomia is a common side effect of radiation therapy (RT) in patients with head and neck cancer. However, limited information is available on the temporal dynamics of parenchymal and vascular changes in salivary glands following RT. To address this gap in knowledge, we conducted experimental studies in mice employing ultrasound (US) with coregistered photoacoustic imaging (PAI) to noninvasively assess the early and late changes in salivary gland size, structure, vascularity, and oxygenation dynamics following RT. Multiparametric US-PAI of salivary glands was performed in immune-deficient and immune-competent mice before and after RT along with correlative sialometry and ex vivo histologic-immunohistochemical validation. US revealed reduction in gland volume and an early increase in vascular resistance postradiation. This was accompanied by a reduction in glandular oxygen consumption on PAI. Imaging data correlated strongly with salivary secretion and histologic evidence of acinar damage. The magnitude and kinetics of radiation response were impacted by host immune status, with immunodeficient mice showing early and more pronounced vascular injury and DNA damage response compared to immunocompetent animals. Our findings demonstrate the ability of noninvasive US-PAI to monitor dynamic changes in salivary gland hemodynamics following radiation and highlight the impact of the host immune status on salivary gland radiation injury.
Keywords: molecular imaging; radiation; saliva; ultrasonography; xerostomia.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L.J. Rich is currently an employee of Fujifilm-VisualSonics Corporation. The remaining authors declare no competing financial interest. The funding sponsors had no role in the design of the study, collection, analyses, or interpretation of data; writing of the manuscript; and the decision to publish the results.
Figures




References
-
- Burdelya LG, Gleiberman AS, Toshkov I, Aygun-Sunar S, Bapardekar M, Manderscheid-Kern P, Bellnier D, Krivokrysenko VI, Feinstein E, Gudkov AV. 2012. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 83(1):228–234. - PMC - PubMed
-
- Chambers MS, Garden AS, Kies MS, Martin JW. 2004. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 26(9):796–807. - PubMed
-
- Cotrim AP, Sowers A, Mitchell JB, Baum BJ. 2007. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 15(12):2101–2106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

