Circ_0000052/miR-382-3p axis induces PD-L1 expression and regulates cell proliferation and immune evasion in head and neck squamous cell carcinoma
- PMID: 36515567
- PMCID: PMC9806294
- DOI: 10.1111/jcmm.17643
Circ_0000052/miR-382-3p axis induces PD-L1 expression and regulates cell proliferation and immune evasion in head and neck squamous cell carcinoma
Abstract
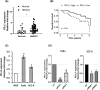
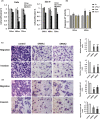
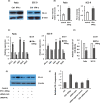
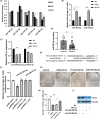
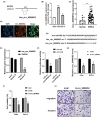
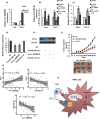
A better understanding of the mechanisms underlying PD-L1 aberrant expression in head and neck squamous cell carcinoma (HNSCC) will help reveal predictive biomarkers and overcome resistance to treatment. In this study, the prognostic significance of PD-L1 in forty-five HNSCC archival samples was determined by qRT-PCR. The biological function associated with malignant behaviour was assessed by PD-L1 depletion, miR-382-3p re-expression and regulation of circ_0000052. The interactions of PD-L1-miRNA and miRNA-circRNA were determined by qRT-PCR, Western blot analysis, dual-luciferase reporter assays and RNA immunoprecipitation assays. PD-L1 was highly expressed in patient samples and cancer cell lines. Higher levels of PD-L1 were associated with patient recurrences and play a pivotal role in regulating cell proliferation, migration, invasion, clonogenicity and apoptosis. In addition to demonstrating that the IFN-γ/JAK2/STAT1 signalling pathway can induce PD-L1 overexpression in HNSCC, a novel mechanism by which upregulated circ_0000052 mediates PD-L1 overexpression was also demonstrated. To do this, circ_0000052 competitively binds to miR-382-3p and alleviates its repression of PD-L1. This leads to overexpression of PD-L1, causing the aggressiveness of the cells. Our data demonstrate that circ_0000052 is oncogenic, and the circ_0000052/miR-382-3p/PD-L1 axis is critical in HNSCC progression. The manipulation of circRNAs/miRNAs in combination with anti-PD-L1 therapy may improve personalized disease management.
Keywords: PD-L1; circular RNA; head and neck squamous cell carcinoma; immunotherapy; microRNA.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

