Prion therapeutics: Lessons from the past
- PMID: 36515657
- PMCID: PMC9754114
- DOI: 10.1080/19336896.2022.2153551
Prion therapeutics: Lessons from the past
Abstract
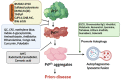
Prion diseases are a group of incurable zoonotic neurodegenerative diseases (NDDs) in humans and other animals caused by the prion proteins. The abnormal folding and aggregation of the soluble cellular prion proteins (PrPC) into scrapie isoform (PrPSc) in the Central nervous system (CNS) resulted in brain damage and other neurological symptoms. Different therapeutic approaches, including stalling PrPC to PrPSc conversion, increasing PrPSc removal, and PrPC stabilization, for which a spectrum of compounds, ranging from organic compounds to antibodies, have been explored. Additionally, a non-PrP targeted drug strategy using serpin inhibitors has been discussed. Despite numerous scaffolds being screened for anti-prion activity in vitro, only a few were effective in vivo and unfortunately, almost none of them proved effective in the clinical studies, most likely due to toxicity and lack of permeability. Recently, encouraging results from a prion-protein monoclonal antibody, PRN100, were presented in the first human trial on CJD patients, which gives a hope for better future for the discovery of other new molecules to treat prion diseases. In this comprehensive review, we have re-visited the history and discussed various classes of anti-prion agents, their structure, mode of action, and toxicity. Understanding pathogenesis would be vital for developing future treatments for prion diseases. Based on the outcomes of existing therapies, new anti-prion agents could be identified/synthesized/designed with reduced toxicity and increased bioavailability, which could probably be effective in treating prion diseases.
Keywords: CJD; Prions disease; anti-prion agents; mode of action; therapeutics; transmissible spongiform encephalopathies.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Prusiner SB, McKinley MP, Bowman KA, et al. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983;35(2):349–358. - PubMed
-
- Caughey B, Kocisko DA, Raymond GJ, et al. Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol. 1995;2(12):807–817. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
