Association of Treatment-Resistant Depression With Patient Outcomes and Health Care Resource Utilization in a Population-Wide Study
- PMID: 36515938
- PMCID: PMC9856735
- DOI: 10.1001/jamapsychiatry.2022.3860
Association of Treatment-Resistant Depression With Patient Outcomes and Health Care Resource Utilization in a Population-Wide Study
Abstract
Importance: The totality of the societal and individual impact of treatment-resistant depression (TRD) is unknown, as is the potential to prognosticate TRD. The generalizability of many observational studies on TRD is limited.
Objective: To estimate the burden of TRD in a large population-wide cohort in an area with universal health care by including data from both health care types (psychiatric and nonpsychiatric) and, further, to develop a prognostic model for clinical use.
Design, setting, and participants: This cohort study, a population-based observational study, assessed data from the Stockholm MDD Cohort for episodes of major depressive disorder (MDD) between 2010 and 2017 that fulfilled predefined criteria for TRD (≥3 consecutive antidepressant treatments). Data analysis was performed from August 2020 to May 2022.
Main outcomes and measures: Outcomes were psychiatric and nonpsychiatric comorbid conditions, antidepressant treatments, health care resource utilization, lost workdays, all-cause mortality, and intentional self-harm and, in the prognostic model, TRD.
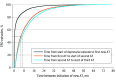
Results: A total of 158 169 unipolar MDD episodes (in 145 577 patients) were identified between January 1, 2012, and December 31, 2017 (64.7% women; median [IQR] age, 42 years [30-56]). Of these, 12 793 episodes (11%) fulfilled criteria for TRD. The median (IQR) time from the start of MDD episode to TRD was 552 days (294-932). Selective serotonin reuptake inhibitor was the most common class of antidepressant treatment in all treatment steps, and 5907 patients (46.2%) received psychotherapy at some point before initiation of the third pharmacological antidepressant treatment. Compared with matched non-TRD episodes, TRD episodes had more inpatient bed-days (mean, 3.9 days; 95% CI, 3.6-4.1, vs 1.3 days; 95% CI, 1.2-1.4) and more lost workdays (mean, 132.3 days; 95% CI, 129.5-135.1, vs 58.7 days; 95% CI, 56.8-60.6) 12 months after the index date. Anxiety, stress, sleep disorder, and substance use disorder were all more common comorbid conditions in TRD episodes. Intentional self-harm was more than 4 times more common in TRD episodes. The all-cause mortality rate for patients with MDD with TRD episodes was 10.7/1000 person-years at risk, compared with 8.7/1000 person-years at risk for patients with MDD without TRD episodes (hazard ratio, 1.23; 95% CI, 1.07-1.41). Median time from start of the first antidepressant treatment to start of the second, and from start of the second antidepressant treatment to start of the third, was 165 and 197 days, respectively. The severity of MDD, defined using the self-rating Montgomery-Åsberg Depression Rating Scale (MADRS-S) at time of MDD diagnosis, was found to be the most important prognostic factor for TRD (C index = 0.69).
Conclusions and relevance: In this cohort study, TRD was a common variant of MDD when including patients from both health care types, which is associated with a high disease burden for both patients and society. The median time between initiation of new antidepressant treatments was longer than recommended in current treatment guidelines, suggesting room for more structured and timely depression care.
Conflict of interest statement
Figures

References
-
- James SL, Abate D, Abate KH, et al. ; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi:10.1016/S0140-6736(18)32279-7 - DOI - PMC - PubMed
-
- Kraepelin E. Manic-depressive insanity and paranoia. J Ment Sci. 1921;67(278):342-346. doi:10.1192/bjp.67.278.342 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

