Outcomes following posttransplant virus-specific T-cell therapy in patients with sickle cell disease
- PMID: 36516084
- PMCID: PMC10196792
- DOI: 10.1182/bloodadvances.2022008219
Outcomes following posttransplant virus-specific T-cell therapy in patients with sickle cell disease
Abstract
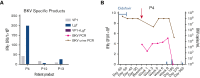
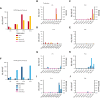
Hematopoietic stem cell transplantation (HSCT) is being increasingly used as a curative approach for sickle cell disease (SCD). With the risk of graft-versus-host disease (GVHD), especially in the human leukocyte antigen-mismatched donors, intense immunosuppression is required leading to an increased risk of viral infection. Post-HSCT, adoptive transfer of virus-specific T-cell (VST) therapies have not been well-studied in patients with SCD. Here, we report the outcomes of patients with SCD at a single-center who received VSTs after transplant to prevent or treat viral infections. Thirteen patients who received HSCT from human leukocyte antigen-matched (n = 9) or -mismatched (n = 4) donors for SCD were treated with a total of 15 VST products for the treatment or prophylaxis of multiple viruses (cytomegalovirus, Epstein-Barr virus, adenovirus, BK virus, human herpes virus 6 +/- human parainfluenza virus 3). Of the patients evaluated, 46.2% (n = 6)) received VSTs as treatment for viral infection. Eighty percent of patients with active viremia (n = 4/5) achieved remission of at least 1 target virus. Seven additional patients (53.8%) received VSTs prophylactically and 6 of 7 (85.7%) remained virus-free after infusion. No immediate infusion-related toxicities occurred, and severe de novo acute GVHD occurred in only 2 (15.4%) patients. Given the good safety profile, high-rate of clinical responses and sustained remissions when administered with standard antiviral treatments, the routine use of VSTs after HSCT as prophylaxis or treatment may improve the overall safety of transplant for patients with SCD.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflicts-of-interest disclosure: M.D.K., A.A., P.J.H., and C.M.B. have intellectual property related to developing T-cell therapies for infectious diseases. C.M.B. has equity interest in Mana Therapeutics and stock or ownership in Cabaletta Bio, Catamaran Bio, Repertoire Immune Medicines, and Neximmune. P.J.H. is a cofounder and on the board of directors of Mana therapeutics, is an advisor to Cellenkos, is on the scientific advisory board of Cellevolve. The remaining authors declare no competing financial interests.
Figures
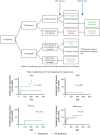
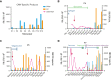
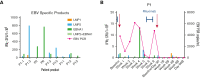
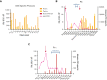

References
-
- Aydin M, Dovern E, Leeflang MMG, et al. Haploidentical allogeneic stem cell transplantation in sickle cell disease: a systematic review and meta-analysis. Transplant Cell Ther. 2021;27(12):1004.e1–1004.e8. - PubMed
-
- Koffi KG, Sawadogo D, Meite M, et al. Reduced levels of T-cell subsets CD4+ and CD8+ in homozygous sickle cell anemia patients with splenic defects. Hematol J. 2003;4(5):363–365. - PubMed
-
- Kharbanda S, Smith AR, Hutchinson SK, et al. Unrelated donor allogeneic hematopoietic stem cell transplantation for patients with hemoglobinopathies using a reduced-intensity conditioning regimen and third-party mesenchymal stromal cells. Biol Blood Marrow Transplant. 2014;20(4):581–586. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

