Transportability Methods for Time-to-Event Outcomes: Application in Adjuvant Colon Cancer Trials
- PMID: 36516368
- PMCID: PMC10166520
- DOI: 10.1200/CCI.22.00088
Transportability Methods for Time-to-Event Outcomes: Application in Adjuvant Colon Cancer Trials
Abstract
Purpose: Differences in the benefits of treatment on 5-year overall survival have been observed in 12 randomized phase III colon cancer adjuvant clinical trials from the ACCENT group. We investigated the reasons for these differences by incorporating the distribution of the observed covariates from each trial.
Materials and methods: We applied state-of-the-art transportability methods on the basis of causal inference, and compared them with a conventional meta-analysis approach to predict the treatment effect for the target population. Prediction errors were defined to evaluate whether the identifiability conditions necessary for causal inference were satisfied among the 12 trials, and to measure the performance of each method.
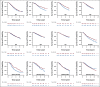
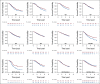
Results: In the one-trial-at-a-time transportability analysis, the ranks of prediction errors for the target population were mostly consistent with the discrepancy in treatment effects among the 12 trials across the three models. The overall prediction errors between the leave-one-trial-out transportability method and the conventional individual participant data meta-analysis approach were very similar, and more than 40% lower than the overall prediction errors from the one-trial-at-a-time transportability method.
Conclusion: The discrepancy in treatment effects among the 12 trials is unlikely to arise from the choice of model specification or distribution of observed covariates but from the distribution of unobserved covariates or study-level features. The ability to quantify heterogeneity among the 12 trials was greatly reduced in both the leave-one-trial-out transportability method and the conventional meta-analysis approach compared with the one-trial-at-a-time transportability method.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–371. - PubMed
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. - PubMed
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. - PubMed
-
- Dahabreh IJ, Robertson SE, Steingrimsson JA, et al. Extending inferences from a randomized trial to a new target population. Stat Med. 2020;39:1999–2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

