Developmental cell fate choice in neural tube progenitors employs two distinct cis-regulatory strategies
- PMID: 36516856
- PMCID: PMC7614300
- DOI: 10.1016/j.devcel.2022.11.016
Developmental cell fate choice in neural tube progenitors employs two distinct cis-regulatory strategies
Abstract
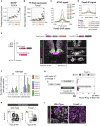
In many developing tissues, the patterns of gene expression that assign cell fate are organized by graded secreted signals. Cis-regulatory elements (CREs) interpret these signals to control gene expression, but how this is accomplished remains poorly understood. In the neural tube, a gradient of the morphogen sonic hedgehog (Shh) patterns neural progenitors. We identify two distinct ways in which CREs translate graded Shh into differential gene expression in mouse neural progenitors. In most progenitors, a common set of CREs control gene activity by integrating cell-type-specific inputs. By contrast, the most ventral progenitors use a unique set of CREs, established by the pioneer factor FOXA2. This parallels the role of FOXA2 in endoderm, where FOXA2 binds some of the same sites. Together, the data identify distinct cis-regulatory strategies for the interpretation of morphogen signaling and raise the possibility of an evolutionarily conserved role for FOXA2 across tissues.
Keywords: Foxa2; chromatin; cis regulation; gene regulation; morphogen; neural tube; sonic hedgehog.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
Two ways to skin a new cell fate.Dev Cell. 2023 Jan 9;58(1):1-2. doi: 10.1016/j.devcel.2022.12.002. Dev Cell. 2023. PMID: 36626868 Free PMC article.
References
-
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba AR, Tanabe Y, Placzek M, Edlund T, Jessell TM. Floor plate and motor neuron induction by vhh-1, a vertebrate ho-molog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

