Evaluating the impact of early identification of asymptomatic brain metastases in metastatic renal cell carcinoma
- PMID: 36517084
- PMCID: PMC10026314
- DOI: 10.1002/cnr2.1763
Evaluating the impact of early identification of asymptomatic brain metastases in metastatic renal cell carcinoma
Abstract
Background: Brain metastases (BM) in metastatic renal cell carcinoma (mRCC) have been reported to be present in up to 25% of patients diagnosed with mRCC. There is limited published literature evaluating the role of routine intra-cranial imaging for the screening of asymptomatic BM in mRCC.
Aims: To evaluate the potential utility of routine intra-cranial imaging, a retrospective cohort study was conducted to characterize the outcomes of mRCC patients who presented with asymptomatic BM, as compared to symptomatic BM.
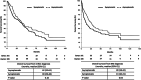
Methods and results: The Canadian Kidney Cancer Information System (CKCis) database was used to identify mRCC patients diagnosed with BM. This cohort was divided into two groups based on the presence or absence of BM symptoms. Details regarding patient demographics, disease characteristics, systemic treatments, BM characteristics and survival outcomes were extracted. Statistical analysis was through chi-square tests, analysis of variance, and Kaplan-Meier method to characterize survival outcomes. A p-value of <0.05 was considered statistically significant for all analyses. A total of 267 mRCC patients with BM were identified of which 106 (40%) presented with asymptomatic disease. The majority of patients presented with multiple (i.e., >1) BM (75%) with no significant differences noted in number of BM or BM-directed therapy received in symptomatic, as compared to asymptomatic BM patients. Median [95% confidence interval (CI)] overall survival (OS) from mRCC diagnosis was 42 months (95% CI: 32-62) for patients with asymptomatic BM, and 39 months (95% CI: 29-48) with symptomatic BM (p = 0.10). OS from time of BM diagnosis was 28 months (95% CI: 18-42) for the asymptomatic BM group, as compared to 13 months (95% CI: 10-21) in the symptomatic BM group (p = 0.04).
Conclusions: Given a substantial proportion of patients may present with asymptomatic BM, limiting intra-cranial imaging to patients with symptomatic BM, may be associated with a missed opportunity for timely diagnosis and treatment. The utility of routine intra-cranial imaging in patients with renal cell carcinoma, warrants further prospective evaluation.
Keywords: brain metastases; imaging; radiation therapy; renal cell carcinoma; surveillance.
© 2022 The Authors. Cancer Reports published by Wiley Periodicals LLC.
Conflict of interest statement
Figures
References
-
- Shuch B, La Rochelle JC, Klatte T, et al. Brain metastasis from renal cell carcinoma: presentation, recurrence, and survival. Cancer. 2008;113:1641‐1648. - PubMed
-
- Ljungberg B, Albiges L, Abu‐Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75:799‐810. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

