Comparison of amyloid burden in individuals with Down syndrome versus autosomal dominant Alzheimer's disease: a cross-sectional study
- PMID: 36517172
- PMCID: PMC9979840
- DOI: 10.1016/S1474-4422(22)00408-2
Comparison of amyloid burden in individuals with Down syndrome versus autosomal dominant Alzheimer's disease: a cross-sectional study
Abstract
Background: Important insights into the early pathogenesis of Alzheimer's disease can be provided by studies of autosomal dominant Alzheimer's disease and Down syndrome. However, it is unclear whether the timing and spatial distribution of amyloid accumulation differs between people with autosomal dominant Alzheimer's disease and those with Down syndrome. We aimed to directly compare amyloid changes between these two groups of people.
Methods: In this cross-sectional study, we included participants (aged ≥25 years) with Down syndrome and sibling controls who had MRI and amyloid PET scans in the first data release (January, 2020) of the Alzheimer's Biomarker Consortium-Down Syndrome (ABC-DS) study. We also included carriers of autosomal dominant Alzheimer's disease genetic mutations and non-carrier familial controls who were within a similar age range to ABC-DS participants (25-73 years) and had MRI and amyloid PET scans at the time of a data freeze (December, 2020) of the Dominantly Inherited Alzheimer Network (DIAN) study. Controls from the two studies were combined into a single group. All DIAN study participants had genetic testing to determine PSEN1, PSEN2, or APP mutation status. APOE genotype was determined from blood samples. CSF samples were collected in a subset of ABC-DS and DIAN participants and the ratio of amyloid β42 (Aβ42) to Aβ40 (Aβ42/40) was measured to evaluate its Spearman's correlation with amyloid PET. Global PET amyloid burden was compared with regards to cognitive status, APOE ɛ4 status, sex, age, and estimated years to symptom onset. We further analysed amyloid PET deposition by autosomal dominant mutation type. We also assessed regional patterns of amyloid accumulation by estimated number of years to symptom onset. Within a subset of participants the relationship between amyloid PET and CSF Aβ42/40 was evaluated.
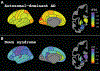
Findings: 192 individuals with Down syndrome and 33 sibling controls from the ABC-DS study and 265 carriers of autosomal dominant Alzheimer's disease mutations and 169 non-carrier familial controls from the DIAN study were included in our analyses. PET amyloid centiloid and CSF Aβ42/40 were negatively correlated in carriers of autosomal dominant Alzheimer's disease mutations (n=216; r=-0·565; p<0·0001) and in people with Down syndrome (n=32; r=-0·801; p<0·0001). There was no difference in global PET amyloid burden between asymptomatic people with Down syndrome (mean 18·80 centiloids [SD 28·33]) versus asymptomatic mutation carriers (24·61 centiloids [30·27]; p=0·11) and between symptomatic people with Down syndrome (77·25 centiloids [41·76]) versus symptomatic mutation carriers (69·15 centiloids [51·10]; p=0·34). APOE ɛ4 status and sex had no effect on global amyloid PET deposition. Amyloid deposition was elevated significantly earlier in mutation carriers than in participants with Down syndrome (estimated years to symptom onset -23·0 vs -17·5; p=0·0002). PSEN1 mutations primarily drove this difference. Early amyloid accumulation occurred in striatal and cortical regions for both mutation carriers (n=265) and people with Down syndrome (n=128). Although mutation carriers had widespread amyloid accumulation in all cortical regions, the medial occipital regions were spared in people with Down syndrome.
Interpretation: Despite minor differences, amyloid PET changes were similar between people with autosomal dominant Alzheimer's disease versus Down syndrome and strongly supported early amyloid dysregulation in individuals with Down syndrome. Individuals with Down syndrome aged at least 35 years might benefit from early intervention and warrant future inclusion in clinical trials, particularly given the relatively high incidence of Down syndrome.
Funding: The National Institute on Aging, Riney and Brennan Funds, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the German Center for Neurodegenerative Diseases, and the Japan Agency for Medical Research and Development.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests TLSB has received funding from the National Institutes of Health and Siemens; has a licensing agreement from Sora Neuroscience but receives no financial compensation; has received honoraria for lectures, presentations, speakers bureaus, or educational events from Biogen and Eisai Genetech; has served on a scientific advisory board for Biogen; holds a leadership role in other board, society, committee, or advocacy groups for the American Society for Neuroradiology (unpaid) and Quantitative Imaging Biomarkers Alliance (unpaid); and has participated in radiopharmaceuticals and technology transfers with Avid Radiopharmaceuticals, Cerveau, and LMI. EMD received support from the National Institute on Aging, an anonymous organisation, the GHR Foundation, the DIAN-TU Pharma Consortium, Eli Lilly, and F Hoffmann La-Roche; has received speaking fees from Eisai and Eli Lilly; and is on the data safety and monitoring board and advisory boards of Eli Lilly, Alector, and Alzamend. WS has received research funding from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. JPC serves as the chair of the American Neurological Association Dementia and Aging Special Interest Group and is on the medical advisory board of Humana Healthcare. CC has received consulting fees from GSK and Alector. AMF reports personal fees from Roche Diagnostics, Araclon/Grifols, and Diadem Research and grants from Biogen, outside the submitted work. BLH has received research funding from Roche and Autism Speaks; receives royalties from Oxford University Press for book publications; and is the chair of the data safety and monitoring board for the Department of Defense-funded study, “Comparative Effectiveness of EIBI and MABA”. BTC receives research funding from the National Institutes of Health. EH receives research funding from the National Institutes of Health and the BrightFocus Foundation. FL is supported by grants from the National Institute on Aging. HDR has received funding from the National Institutes of Health and is on the scientific advisory committee for the Hereditary Disease Foundation. JHL has received research funding from the National Institutes of Health and the National Institute on Aging. RJP receives research funding from the National Institutes of Health and the National Institute on Aging. RJB is Director of DIAN-TU and Principal Investigator of DIAN-TU001; receives research support from the National Institute on Aging of the National Institutes of Health, DIAN-TU trial pharmaceutical partners (Eli Lilly, F Hoffmann-La Roche, Janssen, Eisai, Biogen, and Avid Radiopharmaceuticals), the Alzheimer's Association, the GHR Foundation, an anonymous organisation, the DIAN-TU Pharma Consortium (active members Biogen, Eisai, Eli Lilly, Janssen, and F Hoffmann-La Roche/Genentech; previous members AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, and United Neuroscience), the NfL Consortium (F Hoffmann-La Roche, Biogen, AbbVie, and Bristol Myers Squibb), and the Tau SILK Consortium (Eli Lilly, Biogen, and AbbVie); has been an invited speaker and consultant for AC Immune, F Hoffmann-La Roche, the Korean Dementia Association, the American Neurological Association, and Janssen; has been a consultant for Amgen, F Hoffmann-La Roche, and Eisai; and has submitted the US non-provisional patent application named “Methods for Measuring the Metabolism of CNS Derived Biomolecules In Vivo” and a provisional patent application named “Plasma Based Methods for Detecting CNS Amyloid Deposition”. BMA receives research funding from the National Institutes of Health and has a patent pending (“Markers of Neurotoxicity in CAR T patients”). MSR has received consulting fees from AC Immune, Embic, and Keystone Bio and has received research support from the National Institutes of Health, Avid, Baxter, Eisai, Elan, Genentech, Janssen, Lilly, Merck, and Roche. All other authors declare no competing interests.
Figures
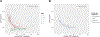

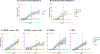

Comment in
-
Lessons from Down syndrome and autosomal dominant Alzheimer's disease.Lancet Neurol. 2023 Jan;22(1):5-6. doi: 10.1016/S1474-4422(22)00437-9. Lancet Neurol. 2023. PMID: 36517171 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- BRC-1215-20014/DH_/Department of Health/United Kingdom
- R33 AG066543/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- K01 AG053474/AG/NIA NIH HHS/United States
- T32 AG058518/AG/NIA NIH HHS/United States
- U01 AG051412/AG/NIA NIH HHS/United States
- UF1 AG032438/AG/NIA NIH HHS/United States
- U01 AG051406/AG/NIA NIH HHS/United States
- P50 HD103525/HD/NICHD NIH HHS/United States
- P30 AG066519/AG/NIA NIH HHS/United States
- U19 AG068054/AG/NIA NIH HHS/United States
- S10 OD025214/OD/NIH HHS/United States
- R01 AG052550/AG/NIA NIH HHS/United States
- R61 AG066543/AG/NIA NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

