CSF P-Tau181 and Other Biomarkers in Patients With Neuronal Intranuclear Inclusion Disease
- PMID: 36517236
- PMCID: PMC9990848
- DOI: 10.1212/WNL.0000000000201647
CSF P-Tau181 and Other Biomarkers in Patients With Neuronal Intranuclear Inclusion Disease
Abstract
Background and objectives: CSF tau phosphorylated at threonine 181 (p-tau181) is a widely used biomarker for Alzheimer disease (AD) and has recently been regarded to reflect β-amyloid and/or p-tau deposition in the AD brain. Neuronal intranuclear inclusion disease (NIID) is a neurodegenerative disease characterized by intranuclear inclusions in neurons, glial cells, and other somatic cells. Symptoms include dementia, neuropathy, and others. CSF biomarkers were not reported. The objective of this study was to investigate whether CSF biomarkers including p-tau181 are altered in patients with NIID.
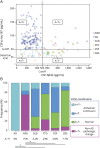
Methods: This was a retrospective observational study. CSF concentrations of p-tau181, total tau, amyloid-beta 1-42 (Aβ42), monoamine metabolites homovanillic acid (HVA), and 5-hydroxyindole acetic acid (5-HIAA) were compared between 12 patients with NIID, 120 patients with Alzheimer clinical syndrome biologically confirmed based on CSF biomarker profiles, and patients clinically diagnosed with other neurocognitive disorders (dementia with Lewy bodies [DLB], 24; frontotemporal dementia [FTD], 13; progressive supranuclear palsy [PSP], 21; and corticobasal syndrome [CBS], 13). Amyloid PET using Pittsburgh compound B (PiB) was performed in 6 patients with NIID.
Results: The mean age of patients with NIID, AD, DLB, FTD, PSP, and CBS was 71.3, 74.6, 76.8, 70.2, 75.5, and 71.9 years, respectively. CSF p-tau181 was significantly higher in NIID (72.7 ± 24.8 pg/mL) compared with DLB, PSP, and CBS and was comparable between NIID and AD. CSF p-tau181 was above the cutoff value (50.0 pg/mL) in 11 of 12 patients with NIID (91.7%). Within these patients, only 2 patients showed decreased CSF Aβ42, and these patients showed negative or mild local accumulation in PiB PET, respectively. PiB PET scans were negative in the remaining 4 patients tested. The proportion of patients with increased CSF p-tau181 and normal Aβ42 (A-T+) was significantly higher in NIID (75%) compared with DLB, PSP, and CBS (4.2%, 4.8%, and 7.7%, respectively). CSF HVA and 5-HIAA concentrations were significantly higher in patients with NIID compared with disease controls.
Discussion: CSF p-tau181 was increased in patients with NIID without amyloid accumulation. Although the deposition of p-tau has not been reported in NIID brains, the molecular mechanism of tau phosphorylation or secretion of p-tau may be altered in NIID.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
