Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer's disease
- PMID: 36517479
- PMCID: PMC9751139
- DOI: 10.1038/s41467-022-34510-3
Optimal deep brain stimulation sites and networks for stimulation of the fornix in Alzheimer's disease
Abstract
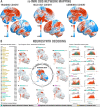
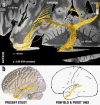
Deep brain stimulation (DBS) to the fornix is an investigational treatment for patients with mild Alzheimer's Disease. Outcomes from randomized clinical trials have shown that cognitive function improved in some patients but deteriorated in others. This could be explained by variance in electrode placement leading to differential engagement of neural circuits. To investigate this, we performed a post-hoc analysis on a multi-center cohort of 46 patients with DBS to the fornix (NCT00658125, NCT01608061). Using normative structural and functional connectivity data, we found that stimulation of the circuit of Papez and stria terminalis robustly associated with cognitive improvement (R = 0.53, p < 0.001). On a local level, the optimal stimulation site resided at the direct interface between these structures (R = 0.48, p < 0.001). Finally, modulating specific distributed brain networks related to memory accounted for optimal outcomes (R = 0.48, p < 0.001). Findings were robust to multiple cross-validation designs and may define an optimal network target that could refine DBS surgery and programming.
© 2022. The Author(s).
Conflict of interest statement
C.N. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG NE 2276/1-1). K.F. received grants and personal fees from Medtronic and Boston Scientific, grants from Abbott/St. Jude, and Functional Neuromodulation outside the submitted work. D.W. received grants from Functional Neuromodulation during conduct of this study, grants and personal fees from Avid/Lily, and Merck, personal fees from Jannsen, GE Healthcare, Biogen and Neuronix outside the submitted work. S.S. receives personal fees from Elsai, Lilly, Roche Novartis and Biogen outside the submitted work. M.S. received personal fees from Allergan, Biogen, Roche-Genentech, Cortexyme, Bracket, Sanofi, and other type of support from Brain Health Inc and uMethod Health outside of the submitted work. C.L. received grants from Functional Neuromodulation Inc. during conduct of this study, from Avanir and Eli Lily and NFL Benefits Office outside of the submitted work. M.O. received grants from NIH, Tourette Association of America Grant, Parkinson’s Alliance, Smallwood Foundation, and personal fees from Parkinson’s Foundation Medical Director, Books4Patients, American Academy of Neurology, Peerview, WebMD/Medscape, Mededicus, Movement Disorders Society, Taylor and Francis, Demos, Robert Rose and non-financial support from Medtronic outside of the submitted work. A.L. received grants from Medtronic and Functional Neuromodulation during conduct of this study, personal fees from Medtronic, St. Jude, Boston Scientific, and Functional Neuromodulation outside of submitted work. A.L. disclosed having a patent “US Patent 8,346,365. Lozano AM. Cognitive function within a human brain. 2013” licensed to Functional Neuromodulation. A.H. was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, 424778381 – TRR 295), Deutsches Zentrum für Luft- und Raumfahrt (DynaSti grant within the EU Joint Programme Neurodegenerative Disease Research, JPND), the National Institutes of Health (R01 13478451, 1R01NS127892-01 & 2R01 MH113929) as well as the New Venture Fund (FFOR Seed Grant). ADvance was supported by the National Institute on Aging (R01AG042165) and Functional Neuromodulation Ltd., the sponsor of the study. Other co-authors report no conflicts of interest.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

