Microglial P2Y12 mediates chronic stress-induced synapse loss in the prefrontal cortex and associated behavioral consequences
- PMID: 36517583
- PMCID: PMC10354016
- DOI: 10.1038/s41386-022-01519-7
Microglial P2Y12 mediates chronic stress-induced synapse loss in the prefrontal cortex and associated behavioral consequences
Abstract
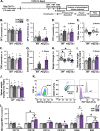
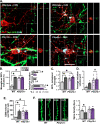
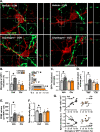
Chronic unpredictable stress (CUS) drives microglia-mediated neuronal remodeling and synapse loss in the prefrontal cortex (PFC), contributing to deficits in cognition and behavior. However, it remains unclear what mechanisms guide microglia-neuron interactions in stress. Evidence indicates that neuronal activity-dependent purinergic signaling directs microglial processes and synaptic engagement via P2Y12, a purinergic receptor exclusively expressed by microglia in the brain. Stress alters excitatory neurotransmission in the PFC, thus we aimed to determine if P2Y12 signaling promotes functional changes in microglia in chronic stress. Here we used genetic ablation of P2Y12 (P2ry12-/-) or pharmacological blockade (clopidogrel, ticagrelor) to examine the role of purinergic signaling in stress-induced microglia-neuron interaction. Multiple behavioral, physiological, and cytometric endpoints were analyzed. Deletion of P2Y12 led to a number of fundamental alterations in the PFC, including the heightened microglial number and increased dendritic spine density. Flow cytometry revealed that microglia in P2ry12-/- mice had shifts in surface levels of CX3CR1, CSF1R, and CD11b, suggesting changes in synaptic engagement and phagocytosis in the PFC. In line with this, pharmacological blockade of P2Y12 prevented CUS-induced increases in the proportion of microglia with neuronal inclusions, limited dendritic spine loss in the PFC, and attenuated alterations in stress coping behavior and working memory function. Overall, these findings indicate that microglial P2Y12 is a critical mediator of stress-induced synapse loss in the PFC and subsequent behavioral deficits.
© 2022. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

