Structural insights into the mechanism of the sodium/iodide symporter
- PMID: 36517601
- PMCID: PMC10501339
- DOI: 10.1038/s41586-022-05530-2
Structural insights into the mechanism of the sodium/iodide symporter
Abstract
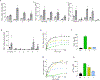
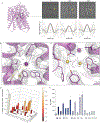
The sodium/iodide symporter (NIS) is the essential plasma membrane protein that mediates active iodide (I-) transport into the thyroid gland, the first step in the biosynthesis of the thyroid hormones-the master regulators of intermediary metabolism. NIS couples the inward translocation of I- against its electrochemical gradient to the inward transport of Na+ down its electrochemical gradient1,2. For nearly 50 years before its molecular identification3, NIS was the molecule at the centre of the single most effective internal radiation cancer therapy: radioiodide (131I-) treatment for thyroid cancer2. Mutations in NIS cause congenital hypothyroidism, which must be treated immediately after birth to prevent stunted growth and cognitive deficiency2. Here we report three structures of rat NIS, determined by single-particle cryo-electron microscopy: one with no substrates bound; one with two Na+ and one I- bound; and one with one Na+ and the oxyanion perrhenate bound. Structural analyses, functional characterization and computational studies show the substrate-binding sites and key residues for transport activity. Our results yield insights into how NIS selects, couples and translocates anions-thereby establishing a framework for understanding NIS function-and how it transports different substrates with different stoichiometries and releases substrates from its substrate-binding cavity into the cytosol.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures





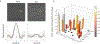



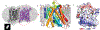
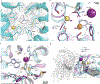
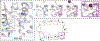
References
-
- Dai G, Levy O & Carrasco N Cloning and characterization of the thyroid iodide transporter. Nature 379, 458–460 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

