Phase 2 study of AV-GBM-1 (a tumor-initiating cell targeted dendritic cell vaccine) in newly diagnosed Glioblastoma patients: safety and efficacy assessment
- PMID: 36517865
- PMCID: PMC9749349
- DOI: 10.1186/s13046-022-02552-6
Phase 2 study of AV-GBM-1 (a tumor-initiating cell targeted dendritic cell vaccine) in newly diagnosed Glioblastoma patients: safety and efficacy assessment
Abstract
Background: Vaccine immunotherapy may improve survival in Glioblastoma (GBM). A multicenter phase II trial was designed to determine: (1) the success rate of manufacturing the Aivita GBM vaccine (AV-GBM-1), (2) Adverse Events (AE) associated with AV-GBM-1 administration, and (3) survival.
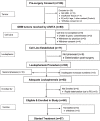
Methods: Fresh suspected glioblastoma tissue was collected during surgery, and patients with pathology-confirmed GBM enrolled before starting concurrent Radiation Therapy and Temozolomide (RT/TMZ) with Intent to Treat (ITT) after recovery from RT/TMZ. AV-GBM-1 was made by incubating autologous dendritic cells with a lysate of irradiated autologous Tumor-Initiating Cells (TICs). Eligible patients were adults (18 to 70 years old) with a Karnofsky Performance Score (KPS) of 70 or greater, a successful TIC culture, and sufficient monocytes collected. A cryopreserved AV-GBM-1 dose was thawed and admixed with 500 μg of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) before every subcutaneous (s.c.) administration.
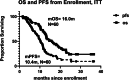
Results: Success rates were 97% for both TIC production and monocyte collection. AV-GBM-1 was manufactured for 63/63 patients; 60 enrolled per ITT; 57 started AV-GBM-1. The most common AEs attributed to AV-GBM-1 were local injection site reactions (16%) and flu-like symptoms (10%). Treatment-emergent AEs included seizures (33%), headache (37%), and focal neurologic symptoms (28%). One patient discontinued AV-GBM-1 because of seizures. Median Progression-Free Survival (mPFS) and median Overall Survival (mOS) from ITT enrollment were 10.4 and 16.0 months, respectively. 2-year Overall Survival (OS) is 27%.
Conclusions: AV-GBM-1 was reliably manufactured. Treatment was well-tolerated, but there were numerous treatment-emergent central nervous system AEs. mPFS was longer than historical benchmarks, though no mOS improvement was noted.
Trial registration: NCT, NCT03400917 , Registered 10 January 2018.
Keywords: Autologous tumor antigens; Dendritic cell vaccine; Glioblastoma; Survival.
© 2022. The Author(s).
Conflict of interest statement
Authors CH, PGB, GIN, HK, PGB and ROD are/were affiliated with Aivita Medical, the sponsor of this clinical trial.
Figures
References
-
- Nabors LB, et al. Central nervous system cancer. National Comprehensive Cancer Network; 2020.