Models of microglia depletion and replenishment elicit protective effects to alleviate vascular and neuronal damage in the diabetic murine retina
- PMID: 36517889
- PMCID: PMC9753268
- DOI: 10.1186/s12974-022-02659-9
Models of microglia depletion and replenishment elicit protective effects to alleviate vascular and neuronal damage in the diabetic murine retina
Abstract
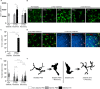
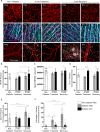
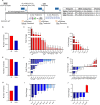
Microglia, the resident phagocytes of the retina, are believed to influence the development of retinopathy, but their exact contributions to vascular integrity and neuronal loss are unknown. Therefore, utilizing two models of microglia depletion, we aimed to deplete and repopulate microglia to clarify the contribution of microglia to neuronal loss and vascular damage in the diabetic retina in an STZ-induced model of hyperglycemia. Here, we report that 2 weeks exposure to diphtheria toxin (DTx) in diabetic CX3CR1CreER:R26iDTR transgenic mice induced a 62% increase in Iba1+ microglia associated with an increase in TUJ1+ axonal density and prevention of NeuN+RBPMS+ neuronal loss. Conversely, diabetic PBS controls exhibited robust TUJ1+ axonal and NeuN+RBPMS+ neuronal loss compared to non-diabetic controls. A 2-week recovery period from DTx was associated with a 40% reduction in angiogenesis and an 85% reduction in fibrinogen deposition into the diabetic retina in comparison to diabetic PBS-treated controls. Analysis of microglia morphology and marker expression revealed that following a 2-week recovery period microglia displayed a P2RY12+Ly6C- phenotype and high transformation index (TI) values complimented by a ramified-surveillant morphology closely resembling non-diabetic controls. In contrast, diabetic PBS-treated control mice displayed P2RY12+Ly6C+ microglia, with a 50% reduction in TI values with an amoeboid morphology. To validate these observations were due to microglia depletion, we used PLX-5622 to assess vascular and neuronal damage in the retinas of diabetic mice. Confocal microscopy revealed that PLX-5622 also induced an increase in TUJ1+ axonal density and prevented fibrinogen extravasation into the diabetic retina. mRNAseq gene expression analysis in retinal isolates revealed that PLX-5622-induced microglia depletion and repopulation induced a downregulation in genes associated with microglial activation and phagocytosis, B2m, Cx3cr1, and Trem2, and complement-associated synaptic pruning, C1qa, C1qb, and C1qc. Although the levels of microglia depletion induced with DTx in the CX3CR1CreER:R26iDTR model and those induced with the CSF-1R antagonists are distinct, our results suggest that microglia depletion and replenishment is neuroprotective by inducing the proliferation of a homeostatic microglia pool that supports neuronal and vascular integrity.
Keywords: Depletion; Diabetic retinopathy; Inflammation; Microglia; Repopulation.
© 2022. The Author(s).
Conflict of interest statement
AEC is an associate editor for the
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

