SNCA genetic lowering reveals differential cognitive function of alpha-synuclein dependent on sex
- PMID: 36517890
- PMCID: PMC9749314
- DOI: 10.1186/s40478-022-01480-y
SNCA genetic lowering reveals differential cognitive function of alpha-synuclein dependent on sex
Erratum in
-
Correction: SNCA genetic lowering reveals differential cognitive function of alpha-synuclein dependent on sex.Acta Neuropathol Commun. 2024 Jun 11;12(1):92. doi: 10.1186/s40478-024-01789-w. Acta Neuropathol Commun. 2024. PMID: 38863027 Free PMC article. No abstract available.
Abstract
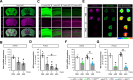
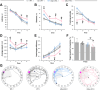
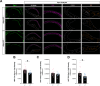
Antisense oligonucleotide (ASO) therapy for neurological disease has been successful in clinical settings and its potential has generated hope for Alzheimer's disease (AD). We previously described that ablating SNCA encoding for α-synuclein (αSyn) in a mouse model of AD was beneficial. Here, we sought to demonstrate whether transient reduction of αSyn expression using ASOSNCA could be therapeutic in a mouse model of AD. The efficacy of the ASOSNCA was measured via immunocytochemistry, RT-qPCR and western blotting. To assess spatial learning and memory, ASOSNCA or PBS-injected APP and non-transgenic (NTG) mice, and separate groups of SNCA-null mice, were tested on the Barnes circular maze. Hippocampal slice electrophysiology and transcriptomic profiling were used to explore synaptic function and differential gene expression between groups. Reduction of SNCA transcripts alleviated cognitive deficits in male transgenic animals, but surprisingly, not in females. To determine the functional cause of this differential effect, we assessed memory function in SNCA-null mice. Learning and memory were intact in male mice but impaired in female animals, revealing that the role of αSyn on cognitive function is sex-specific. Transcriptional analyses identified a differentially expressed gene network centered around EGR1, a central modulator of learning and memory, in the hippocampi of SNCA-null mice. Thus, these novel results demonstrate that the function of αSyn on memory differs between male and female brains.
Keywords: Alpha-synuclein; Alzheimer’s disease; Antisense oligonucleotide; Early growth response 1; Sex; Spatial memory; Synucleinopathy.
© 2022. The Author(s).
Conflict of interest statement
Unless stated subsequently, the authors declare that they have no competing interests. T. Cole: Other Research Support (receipt of drugs, supplies, equipment or other in-kind support); Provided drug (mouse alpha-synuclein ASO).
Figures


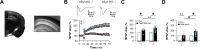

References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Garcia Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. - DOI - PubMed
-
- Alarcón-Arís D, Pavia-Collado R, Miquel-Rio L, Coppola-Segovia V, Ferrés-Coy A, Ruiz-Bronchal E, Galofré M, Paz V, Campa L, Revilla R, Montefeltro A, Kordower JH, Vila M, Artigas F, Bortolozzi A. Anti-α-synuclein ASO delivered to monoamine neurons prevents α-synuclein accumulation in a Parkinson’s disease-like mouse model and in monkeys. EBioMedicine. 2020;59:102944. doi: 10.1016/J.EBIOM.2020.102944. - DOI - PMC - PubMed
-
- Bakalash S, Pham M, Koronyo Y, Salumbides BC, Kramerov A, Seidenberg H, Berel D, Black KL, Koronyo-Hamaoui M. Egr1 expression is induced following glatiramer acetate immunotherapy in rodent models of glaucoma and alzheimer’s disease. Invest Ophthalmol Vis Sci. 2011;52:9033–9046. doi: 10.1167/IOVS.11-7498. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG077743/AG/NIA NIH HHS/United States
- R01 NS086074/NS/NINDS NIH HHS/United States
- T32 NS105604/NS/NINDS NIH HHS/United States
- R01 NS092093/NS/NINDS NIH HHS/United States
- R56 AG074473/AG/NIA NIH HHS/United States
- R56 NS113549/NS/NINDS NIH HHS/United States
- RF1 AG062135/AG/NIA NIH HHS/United States
- R01 DA048822/DA/NIDA NIH HHS/United States
- RF1 AG044342/AG/NIA NIH HHS/United States
- R01 AG044342/AG/NIA NIH HHS/United States
- F31 NS125963/NS/NINDS NIH HHS/United States
- RF1 AG070296/AG/NIA NIH HHS/United States
- R21 AG065693/AG/NIA NIH HHS/United States
- R01 NS092918/NS/NINDS NIH HHS/United States
- R01 AG020135/AG/NIA NIH HHS/United States
- R01 NS108686/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

