An induced pluripotent stem cell-based model identifies molecular targets of vincristine neurotoxicity
- PMID: 36518084
- PMCID: PMC10655812
- DOI: 10.1242/dmm.049471
An induced pluripotent stem cell-based model identifies molecular targets of vincristine neurotoxicity
Abstract
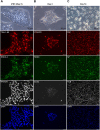
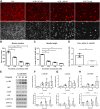
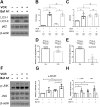
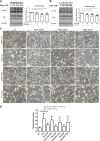
To model peripheral nerve degeneration and investigate molecular mechanisms of neurodegeneration, we established a cell system of induced pluripotent stem cell (iPSC)-derived sensory neurons exposed to vincristine, a drug that frequently causes chemotherapy-induced peripheral neuropathy. Sensory neurons differentiated from iPSCs exhibit distinct neurochemical patterns according to the immunocytochemical phenotypes, and gene expression of peripherin (PRPH, hereafter referred to as Peri) and neurofilament heavy chain (NEFH, hereafter referred to as NF). The majority of iPSC-derived sensory neurons were PRPH positive/NEFH negative, i.e. Peri(+)/NF(-) neurons, whose somata were smaller than those of Peri(+)/NF(+) neurons. On exposure to vincristine, projections from the cell body of a neuron, i.e. neurites, were degenerated quicker than somata, the lethal concentration to kill 50% (LC50) of neurites being below the LC50 for somata, consistent with the clinical pattern of length-dependent neuropathy. We then examined the molecular expression in the MAP kinase signaling pathways of, extracellular signal-regulated kinases 1/2 (MAPK1/3, hereafter referred to as ERK), p38 mitogen-activated protein kinases (MAPK11/12/13/14, hereafter referred to as p38) and c-Jun N-terminal kinases (MAPK8/9/10, hereafter referred to as JNK). Regarding these three cascades, only phosphorylation of JNK was upregulated but not that of p38 or ERK1/2. Furthermore, vincristine-treatment resulted in impaired autophagy and reduced autophagic flux. Rapamycin-treatment reversed the effect of impaired autophagy and JNK activation. These results not only established a platform to study peripheral degeneration of human neurons but also provide molecular mechanisms for neurodegeneration with the potential for therapeutic targets.
Keywords: Autophagy; IPSC; MAP kinase; Nerve degeneration; Sensory neuron; Vincristine.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

