Lecithin-based nanocapsule loading sucupira (Pterodon emarginatus) oil effects in experimental mucositis
- PMID: 36518414
- PMCID: PMC9743464
- DOI: 10.1016/j.toxrep.2022.07.006
Lecithin-based nanocapsule loading sucupira (Pterodon emarginatus) oil effects in experimental mucositis
Abstract
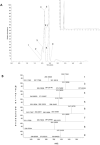
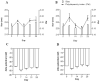
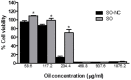
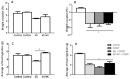
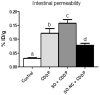
Intestinal mucositis (IM) is a frequent adverse effect in anticancer therapy without standard treatment. The oil obtained from sucupira (Pterodon emarginatus) has anti-inflammatory properties, and the soybean lecithin reduces the intestinal toxicity of several xenobiotics. However, their water insolubility impairs the in vivo application. For this reason, we evaluated if the nanoencapsulation of sucupira oil (SO) in lecithin-based nanocapsules (SO-NC) could be a therapeutically effective system for the treatment of IM in murine cisplatin (CDDP)-induced intestinal mucositis model. SO was analyzed by LC-HRMS/MS and HPLC. SO-NC was prepared by nanoprecipitation and characterized using DLS, HPLC, and AFM. Mice body weight and food consumption were assessed daily during experimental mucositis induced by CDDP. The animals were euthanized, and intestinal permeability, inflammatory mediators, and intestinal histology were performed. SO-NC demonstrated adequate characteristics for oral administration as size under 300 nm, IP < 0.3, high EE, and spherical shape. In vitro cytotoxicity performed against RAW 264.7 cell lines resulted in cell viability above 80 % confirming the non-cytotoxic profile of SO (IC50 268 µg/mL) and SO-NC (IC50 118.5 µg/mL) up to 117.2 µg/mL. The untreated mice showed intestinal toxicity after i.p. of CDDP, principally weight loss, increased intestinal permeability, and MPO and TNF-α levels. Surprisingly, the administration of SO to CDDP-mucositis animals did not circumvent the CDDP effects and increased intestinal permeability. However, SO-NC proved efficient in mitigating the experimental intestinal mucositis by improving intestinal epithelium architecture, reducing intestinal permeability, and improving the MPO levels. In conclusion, SO-NC can positively impact intestinal mucositis by promoting mucosal recovery. This is a promising strategy for developing a new treatment for intestinal mucositis.
Keywords: Intestinal permeability; Lecithin; Mucositis; Nanocapsules; Pterodon emarginatus.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Sonis S.T. The pathobiology of mucositis. Nat Rev Cancer. Published online 2004. doi:10.1038/nrc1318. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

