In vitro and in vivo hepatotoxicity study of Afriplex™ GRT through an inflammatory response
- PMID: 36518449
- PMCID: PMC9742939
- DOI: 10.1016/j.toxrep.2022.10.006
In vitro and in vivo hepatotoxicity study of Afriplex™ GRT through an inflammatory response
Abstract
Background: The focus on traditional and complementary medicine for supplementation and treatment of diseases is high. Aspalathus linearis commonly known as Rooibos showed several beneficial effects, this led to the standardized production of a pharmaceutical grade green rooibos extract (Afriplex TM GRT) with enhanced polyphenolic content. The aim of this study was to assess toxicity of Afriplex TM GRT in HepG2/C3A cells and Sprague Dawley rats.
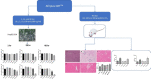
Methods: Afriplex GRT TM (0.1, 1, 10, 100, or 1000 μg/mL) in DMSO was added to the media to the final 0.01% DMSO for treatment of HepG2/C3A for 1, 24 and 48 hrs followed by MTT and ATP assays. Sprague Dawley rats were grouped to Control, Afriplex TM GRT treated (10, 100 and 300 mg/kg); and acute (24hrs tetrachloromethane (CCl 4) injected hepatotoxicity control). Serum biochemistry, histology and Western blot analysis on liver were performed.
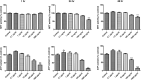
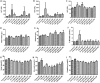
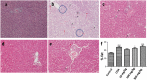
Results: Afriplex TM GRT significantly reduced cell viability at 100 and 1000μg/mL after 48 hrs. Acute CCl 4 treatment significantly increased serum alanine aminotransferase in rats. The highest extract treatment of 300 mg/kg significantly elevated aspartate amino transferase. There was severe macro vesicular in the CCl 4 group whereas mild to moderate micro vesicular steatosis was seen in the 300 mg/kg Afriplex TM GRT treated group. Highest extract treatment significantly reduced NFkB expression on Western blot analysis.
Conclusion: The beneficial effects of pharmaceutical grade Afriplex GRT TM are concentration and dosage based. Afriplex GRT TM exerts its beneficial effects via NFkB as demonstrated by the dose dependent reduction of NFkB on Western blot analysis. More work need to be done to explore the exact mechanism that occurs in the NFkB pathway.
Keywords: Aspalathin-rich; HepG2/C3A; Hepatotoxicity; Rooibos; Sprague Dawley.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- El-Sayed A.M.H. Possible effects of natural antioxidants on experimentally induced hepatotoxicity in rats. CU Theses. 2016;0
-
- Navarro V., Senior J. Drug-related hepatotoxicity. N. Engl. J. Med. 2006;354:731–739. - PubMed
-
- Leise M.D., Poterucha J.J., Talwalkar J.A. Drug-induced liver injury. Mayo Clin. Proc. 2014;89:95–106. - PubMed
-
- Björnsson E.S. Drug-induced liver injury: an overview over the most critical compounds. Arch. Toxicol. 2015;89:327–334. - PubMed
LinkOut - more resources
Full Text Sources

