From tryptamine to the discovery of efficient multi-target directed ligands against cholinesterase-associated neurodegenerative disorders
- PMID: 36518670
- PMCID: PMC9742383
- DOI: 10.3389/fphar.2022.1036030
From tryptamine to the discovery of efficient multi-target directed ligands against cholinesterase-associated neurodegenerative disorders
Abstract
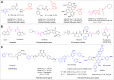
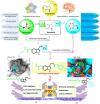
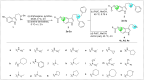
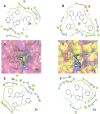
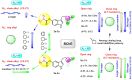
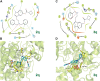
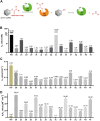
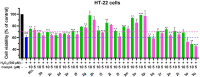
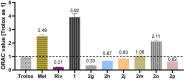
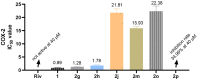
A novel class of benzyl-free and benzyl-substituted carbamylated tryptamine derivatives (CDTs) was designed and synthesized to serve as effective building blocks for the development of novel multi-target directed ligands (MTDLs) for the treatment of neurological disorders linked to cholinesterase (ChE) activity. The majority of them endowed butyrylcholinesterase (BuChE) with more substantial inhibition potency than acetylcholinesterase (AChE), according to the full study of ChE inhibition. Particularly, hybrids with dibenzyl groups (2b-2f, 2j, 2o, and 2q) showed weak or no neuronal toxicity and hepatotoxicity and single-digit nanomolar inhibitory effects against BuChE. Through molecular docking and kinetic analyses, the potential mechanism of action on BuChE was first investigated. In vitro H2O2-induced HT-22 cells assay demonstrated the favorable neuroprotective potency of 2g, 2h, 2j, 2m, 2o, and 2p. Besides, 2g, 2h, 2j, 2m, 2o, and 2p endowed good antioxidant activities and COX-2 inhibitory effects. This study suggested that this series of hybrids can be applied to treat various ChE-associated neurodegenerative disorders such as Alzheimer's disease (AD) and Parkinson's disease (PD), as well as promising building blocks for further structure modification to develop efficient MTDLs.
Keywords: MTDLs; anti-neuroinflammation; benzylation; carbamylated tryptamine derivatives; cholinesterase inhibitors; neurodegenerative disorders; neuroprotection; promising building blocks.
Copyright © 2022 Wu, Zhang, Wang, Yin, Li, Zhuo, Chen and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

