Effect of M6A regulators on diagnosis, subtype classification, prognosis and novel therapeutic target development of idiopathic pulmonary fibrosis
- PMID: 36518679
- PMCID: PMC9742476
- DOI: 10.3389/fphar.2022.993567
Effect of M6A regulators on diagnosis, subtype classification, prognosis and novel therapeutic target development of idiopathic pulmonary fibrosis
Erratum in
-
Corrigendum: Effect of M6A regulators on diagnosis, subtype classification, prognosis and novel therapeutic target development of idiopathic pulmonary fibrosis.Front Pharmacol. 2022 Dec 16;13:1117317. doi: 10.3389/fphar.2022.1117317. eCollection 2022. Front Pharmacol. 2022. PMID: 36588674 Free PMC article.
Abstract
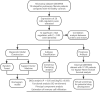
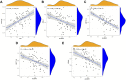
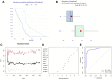
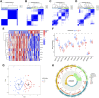
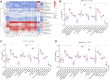
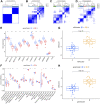
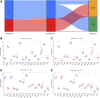
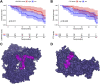
Molecular biology studies show that RNA N6-methyladenosine (m6A) modifications may take part in the incidence and development of idiopathic pulmonary fibrosis (IPF). Nonetheless, the roles of m6A regulators in IPF are not fully demonstrated. In this study, 12 significant m6A regulators were filtered out between healthy controls and IPF patients using GSE33566 dataset. Random forest algorithm was used to identify 11 candidate m6A regulators to predict the incidence of IPF. The 11 candidate m6A regulators included leucine-rich PPR motif-containing protein (LRPPRC), methyltransferase-like protein 3, FTO alpha-ketoglutarate dependent dioxygenase (FTO), methyltransferase-like 14/16, zinc finger CCCH domain-containing protein 13, protein virilizer homolog, Cbl proto-oncogene like 1, fragile X messenger ribonucleoprotein 1 and YTH domain containing 1/2. A nomogram model was constructed based on 11 candidate m6A regulators and considered beneficial to IPF patients using decision curve analysis. Consensus clustering method was used to distinctly divide IPF patients into two m6A patterns (clusterA and clusterB) based on 12 significant m6A regulators. M6A scores of all IPF patients were obtained using principal component analysis to quantify the m6A patterns. Patients in clusterB had higher m6A scores than those in clusterA. Furthermore, patients in clusterB were correlated with Th17 and Treg cell infiltration, innate immunity and Th1 immunity, while those in clusterA were correlated with adaptive immunity and Th2 immunity. Patients in clusterB also had higher expressions of mesenchymal markers and regulatory factors of fibrosis but lower expressions of epithelial markers. Lastly and interestingly, two m6A regulators, LRPPRC (p = 0.011) and FTO (p = 0.042), were identified as novel prognostic genes in IPF patients for the first time using an external GSE93606 dataset. Both of them had a positive correlation with a better prognosis and may serve as therapy targets. Thus, we conducted virtual screening to discover potential drugs targeting LRPPRC and FTO in the treatment of IPF. In conclusion, m6A regulators are crucial to the onset, development and prognosis of IPF. Our study on m6A patterns may provide clues for clinical diagnosis, prognosis and targeted therapeutic drugs development for IPF.
Keywords: consensus clustering; diagnostic model; idiopathic pulmonary fibrosis; m6A regulators; novel therapeutic targets; prognostic markers.
Copyright © 2022 Huang, Huang and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

