Case Report: Long-term observations from the tacrolimus weaning randomized clinical trial depicts the challenging aspects for determination of low-immunological risk patients
- PMID: 36518770
- PMCID: PMC9744190
- DOI: 10.3389/fimmu.2022.1021481
Case Report: Long-term observations from the tacrolimus weaning randomized clinical trial depicts the challenging aspects for determination of low-immunological risk patients
Abstract
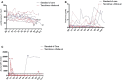
Whilst calcineurin inhibitors (CNI) are the cornerstone of immunosuppressive maintenance therapy in kidney transplantation, several studies have investigated the safety of CNI withdrawal in order to avoid their numerous side effects. In this context, we performed several years ago a clinical randomized trial evaluating CNI weaning in stable kidney transplant recipients without anti-HLA immunization. The trial was interrupted prematurely due to a high number of de novo DSA (dnDSA) and biopsy proven acute rejection (BPAR) in patients who underwent tacrolimus weaning, resulting in treatment for rejection and resumption of tacrolimus. We report here the long-term outcomes of patients included in this clinical trial. Ten years after randomization, all patients are alive with a functional allograft. They all receive tacrolimus therapy except one with recurrent cutaneous neoplasia issues. Long-term eGFR was comparable between patients of the two randomized groups (46.4 ml/min vs 42.8 ml/min). All dnDSA that occurred during the study period became non-detectable and all rejections episodes were reversed. The retrospective assessment of HLA DQ single molecule epitope mismatching determined that a majority of patients who developed dnDSA after tacrolimus withdrawal would have been considered at high immunological risk. Minimization of immunosuppression remains a challenging objective, mainly because of the issues to properly select very low immunological risk patients. Valuable improvements have been made the last decade regarding evaluation of the allograft rejection notably through the determination of numerous at-risk biomarkers. However, even if the impact of such tools still need to be clarify in clinical routine, they may permit an improvement in patients' selection for immunosuppression minimization without increasing the risk of allograft rejection.
Trial registration: ClinicalTrials.gov NCT01292525.
Keywords: allograft rejection; calcineurin inhibitor withdrawal; case report; donor specific antibodies; kidney transplantation.
Copyright © 2022 Masset, Dantal, Soulillou, Walencik, Delbos, Brouard, Giral and the Nantes DIVAT Consortium.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cantarovich D, Rostaing L, Kamar N, Ducloux D, Saint-Hillier Y, Mourad G, et al. . Early corticosteroid avoidance in kidney transplant recipients receiving ATG-f induction: 5-year actual results of a prospective and randomized study. Am J Transpl (2014) 14:2556–64. doi: 10.1111/ajt.12866 - DOI - PubMed
-
- Thomusch O, Wiesener M, Opgenoorth M, Pascher A, Woitas RP, Witzke O, et al. . Rabbit-ATG or basiliximab induction for rapid steroid withdrawal after renal transplantation (Harmony): an open-label, multicentre, randomised controlled trial. Lancet Lond Engl (2016) 388:3006–16. doi: 10.1016/S0140-6736(16)32187-0 - DOI - PubMed
-
- Kampf C, Lau T, Olsson R, Leung PS, Carlsson P-O. Angiotensin II type 1 receptor inhibition markedly improves the blood perfusion, oxygen tension and first phase of glucose-stimulated insulin secretion in revascularised syngeneic mouse islet grafts. Diabetologia (2005) 48:1159–67. doi: 10.1007/s00125-005-1761-z - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

