The copper transporter CTR1 and cisplatin accumulation at the single-cell level by LA-ICP-TOFMS
- PMID: 36518851
- PMCID: PMC9742377
- DOI: 10.3389/fmolb.2022.1055356
The copper transporter CTR1 and cisplatin accumulation at the single-cell level by LA-ICP-TOFMS
Abstract
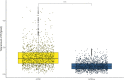
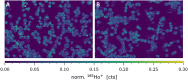
More than a decade ago, studies on cellular cisplatin accumulation via active membrane transport established the role of the high affinity copper uptake protein 1 (CTR1) as a main uptake route besides passive diffusion. In this work, CTR1 expression, cisplatin accumulation and intracellular copper concentration was assessed for single cells revisiting the case of CTR1 in the context of acquired cisplatin resistance. The single-cell workflow designed for in vitro experiments enabled quantitative imaging at resolutions down to 1 µm by laser ablation-inductively coupled plasma-time-of-flight mass spectrometry (LA-ICP-TOFMS). Cisplatin-sensitive ovarian carcinoma cells A2780 as compared to the cisplatin-resistant subline A2780cis were investigated. Intracellular cisplatin and copper levels were absolutely quantified for thousands of individual cells, while for CTR1, relative differences of total CTR1 versus plasma membrane-bound CTR1 were determined. A markedly decreased intracellular cisplatin concentration accompanied by reduced copper concentrations was observed for single A2780cis cells, along with a distinctly reduced (total) CTR1 level as compared to the parental cell model. Interestingly, a significantly different proportion of plasma membrane-bound versus total CTR1 in untreated A2780 as compared to A2780cis cells was observed. This proportion changed in both models upon cisplatin exposure. Statistical analysis revealed a significant correlation between total and plasma membrane-bound CTR1 expression and cisplatin accumulation at the single-cell level in both A2780 and A2780cis cells. Thus, our study recapitulates the crosstalk of copper homeostasis and cisplatin uptake, and also indicates a complex interplay between subcellular CTR1 localization and cellular cisplatin accumulation as a driver for acquired resistance development.
Keywords: CTR1 copper transporter; ICP-TOFMS; cisplatin accumulation; cisplatin resistance; laser ablation; single-cell.
Copyright © 2022 Schoeberl, Gutmann, Theiner, Corte-Rodríguez, Braun, Vician, Berger and Koellensperger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bandura D. R., Baranov V. I., Ornatsky O. I., Antonov A., Kinach R., Lou X., et al. (2009). Mass cytometry: Technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem. 81 (16), 6813–6822. 10.1021/ac901049w - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

