Recent advances in regenerative biomaterials
- PMID: 36518879
- PMCID: PMC9745784
- DOI: 10.1093/rb/rbac098
Recent advances in regenerative biomaterials
Abstract
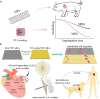
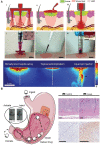
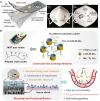
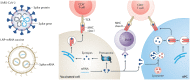
Nowadays, biomaterials have evolved from the inert supports or functional substitutes to the bioactive materials able to trigger or promote the regenerative potential of tissues. The interdisciplinary progress has broadened the definition of 'biomaterials', and a typical new insight is the concept of tissue induction biomaterials. The term 'regenerative biomaterials' and thus the contents of this article are relevant to yet beyond tissue induction biomaterials. This review summarizes the recent progress of medical materials including metals, ceramics, hydrogels, other polymers and bio-derived materials. As the application aspects are concerned, this article introduces regenerative biomaterials for bone and cartilage regeneration, cardiovascular repair, 3D bioprinting, wound healing and medical cosmetology. Cell-biomaterial interactions are highlighted. Since the global pandemic of coronavirus disease 2019, the review particularly mentions biomaterials for public health emergency. In the last section, perspectives are suggested: (i) creation of new materials is the source of innovation; (ii) modification of existing materials is an effective strategy for performance improvement; (iii) biomaterial degradation and tissue regeneration are required to be harmonious with each other; (iv) host responses can significantly influence the clinical outcomes; (v) the long-term outcomes should be paid more attention to; (vi) the noninvasive approaches for monitoring in vivo dynamic evolution are required to be developed; (vii) public health emergencies call for more research and development of biomaterials; and (viii) clinical translation needs to be pushed forward in a full-chain way. In the future, more new insights are expected to be shed into the brilliant field-regenerative biomaterials.
Keywords: medical material; regenerative biomaterial; tissue induction biomaterial; tissue regeneration.
© The Author(s) 2022. Published by Oxford University Press.
Figures








References
-
- Langer R, Vacanti JP.. Tissue engineering. Science 1993;260:920–6. - PubMed
-
- Fu X, Peppas NA, Gu X.. Biomedical Materials and Tissue Regeneration. Beijing: People’s Health Press, 2020.
-
- Hasani-Sadrabadi MM, Sarrion P, Pouraghaei S, Chau Y, Ansari S, Li S, Aghaloo T, Moshaverinia A.. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med 2020;12:eaay6853. - PubMed
-
- Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A.. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol 2016;34:470–82. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

