Real-world Evidence of the Effects of Novel Treatments for COVID-19 on Mortality: A Nationwide Comparative Cohort Study of Hospitalized Patients in the First, Second, Third, and Fourth Waves in the Netherlands
- PMID: 36519114
- PMCID: PMC9745783
- DOI: 10.1093/ofid/ofac632
Real-world Evidence of the Effects of Novel Treatments for COVID-19 on Mortality: A Nationwide Comparative Cohort Study of Hospitalized Patients in the First, Second, Third, and Fourth Waves in the Netherlands
Abstract
Background: Large clinical trials on drugs for hospitalized coronavirus disease 2019 (COVID-19) patients have shown significant effects on mortality. There may be a discrepancy with the observed real-world effect. We describe the clinical characteristics and outcomes of hospitalized COVID-19 patients in the Netherlands during 4 pandemic waves and analyze the association of the newly introduced treatments with mortality, intensive care unit (ICU) admission, and discharge alive.
Methods: We conducted a nationwide retrospective analysis of hospitalized COVID-19 patients between February 27, 2020, and December 31, 2021. Patients were categorized into waves and into treatment groups (hydroxychloroquine, remdesivir, neutralizing severe acute respiratory syndrome coronavirus 2 monoclonal antibodies, corticosteroids, and interleukin [IL]-6 antagonists). Four types of Cox regression analyses were used: unadjusted, adjusted, propensity matched, and propensity weighted.
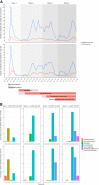
Results: Among 5643 patients from 11 hospitals, we observed a changing epidemiology during 4 pandemic waves, with a decrease in median age (67-64 years; P < .001), in in-hospital mortality on the ward (21%-15%; P < .001), and a trend in the ICU (24%-16%; P = .148). In ward patients, hydroxychloroquine was associated with increased mortality (1.54; 95% CI, 1.22-1.96), and remdesivir was associated with a higher rate of discharge alive within 29 days (1.16; 95% CI, 1.03-1.31). Corticosteroids were associated with a decrease in mortality (0.82; 95% CI, 0.69-0.96); the results of IL-6 antagonists were inconclusive. In patients directly admitted to the ICU, hydroxychloroquine, corticosteroids, and IL-6 antagonists were not associated with decreased mortality.
Conclusions: Both remdesivir and corticosteroids were associated with better outcomes in ward patients with COVID-19. Continuous evaluation of real-world treatment effects is needed.
Keywords: COVID-19; SARS-CoV-2; antiviral; epidemiology; immunosuppressive treatments.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. H.P.S. received a personal grant from the Dutch Kidney Foundation. D.D. and N.K. are chairs of the NICE foundation (unpaid). N.K. processes data from all ICUs into the NICE database; the payment is received by the host institution. A.V. received study support and consultation fees from InflaRx, paid to the host institution. W.J.W. has performed ad hoc consultancies for Sonic, Pfizer, GSK, and AstraZeneca; all fees were paid to the host institution. L.v.V. is supported by the Dutch Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek NOW), ZonMW; VENI grant 09150161910033; and a European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Research Grant. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 324:782–93. - PubMed
-
- RIVM . Coronadashboard. Secondary Coronadashboard. 2022. Available at: https://coronadashboard.rijksoverheid.nl/. Accessed August 8, 2022.
LinkOut - more resources
Full Text Sources
Miscellaneous

