Intellectual enrichment and genetic modifiers of cognition and brain volume in Huntington's disease
- PMID: 36519153
- PMCID: PMC9732861
- DOI: 10.1093/braincomms/fcac279
Intellectual enrichment and genetic modifiers of cognition and brain volume in Huntington's disease
Abstract
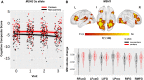
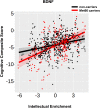
An important step towards the development of treatments for cognitive impairment in ageing and neurodegenerative diseases is to identify genetic and environmental modifiers of cognitive function and understand the mechanism by which they exert an effect. In Huntington's disease, the most common autosomal dominant dementia, a small number of studies have identified intellectual enrichment, i.e. a cognitively stimulating lifestyle and genetic polymorphisms as potential modifiers of cognitive function. The aim of our study was to further investigate the relationship and interaction between genetic factors and intellectual enrichment on cognitive function and brain atrophy in Huntington's disease. For this purpose, we analysed data from Track-HD, a multi-centre longitudinal study in Huntington's disease gene carriers and focused on the role of intellectual enrichment (estimated at baseline) and the genes FAN1, MSH3, BDNF, COMT and MAPT in predicting cognitive decline and brain atrophy. We found that carrying the 3a allele in the MSH3 gene had a positive effect on global cognitive function and brain atrophy in multiple cortical regions, such that 3a allele carriers had a slower rate of cognitive decline and atrophy compared with non-carriers, in agreement with its role in somatic instability. No other genetic predictor had a significant effect on cognitive function and the effect of MSH3 was independent of intellectual enrichment. Intellectual enrichment also had a positive effect on cognitive function; participants with higher intellectual enrichment, i.e. those who were better educated, had higher verbal intelligence and performed an occupation that was intellectually engaging, had better cognitive function overall, in agreement with previous studies in Huntington's disease and other dementias. We also found that intellectual enrichment interacted with the BDNF gene, such that the positive effect of intellectual enrichment was greater in Met66 allele carriers than non-carriers. A similar relationship was also identified for changes in whole brain and caudate volume; the positive effect of intellectual enrichment was greater for Met66 allele carriers, rather than for non-carriers. In summary, our study provides additional evidence for the beneficial role of intellectual enrichment and carrying the 3a allele in MSH3 in cognitive function in Huntington's disease and their effect on brain structure.
Keywords: Huntington’s disease; MSH3; brain-derived neurotrophic factor; cognitive modifiers; intellectual enrichment.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
