Delivery of costimulatory blockade to lymph nodes promotes transplant acceptance in mice
- PMID: 36519543
- PMCID: PMC9754003
- DOI: 10.1172/JCI159672
Delivery of costimulatory blockade to lymph nodes promotes transplant acceptance in mice
Abstract
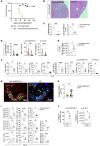
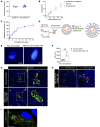
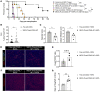
The lymph node (LN) is the primary site of alloimmunity activation and regulation during transplantation. Here, we investigated how fibroblastic reticular cells (FRCs) facilitate the tolerance induced by anti-CD40L in a murine model of heart transplantation. We found that both the absence of LNs and FRC depletion abrogated the effect of anti-CD40L in prolonging murine heart allograft survival. Depletion of FRCs impaired homing of T cells across the high endothelial venules (HEVs) and promoted formation of alloreactive T cells in the LNs in heart-transplanted mice treated with anti-CD40L. Single-cell RNA sequencing of the LNs showed that anti-CD40L promotes a Madcam1+ FRC subset. FRCs also promoted the formation of regulatory T cells (Tregs) in vitro. Nanoparticles (NPs) containing anti-CD40L were selectively delivered to the LNs by coating them with MECA-79, which binds to peripheral node addressin (PNAd) glycoproteins expressed exclusively by HEVs. Treatment with these MECA-79-anti-CD40L-NPs markedly delayed the onset of heart allograft rejection and increased the presence of Tregs. Finally, combined MECA-79-anti-CD40L-NPs and rapamycin treatment resulted in markedly longer allograft survival than soluble anti-CD40L and rapamycin. These data demonstrate that FRCs are critical to facilitating costimulatory blockade. LN-targeted nanodelivery of anti-CD40L could effectively promote heart allograft acceptance.
Keywords: Organ transplantation; Transplantation.
Figures


Comment in
- Fibroblastic reticular cells orchestrate long-term graft survival following recipient treatment with CD40 ligand-targeted costimulatory blockade

