Thalamocortical circuits drive remifentanil-induced postoperative hyperalgesia
- PMID: 36519547
- PMCID: PMC9754001
- DOI: 10.1172/JCI158742
Thalamocortical circuits drive remifentanil-induced postoperative hyperalgesia
Abstract
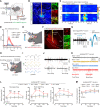
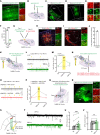
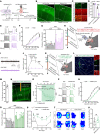
Remifentanil-induced hyperalgesia (RIH) is a severe but common postoperative clinical problem with elusive underlying neural mechanisms. Here, we discovered that glutamatergic neurons in the thalamic ventral posterolateral nucleus (VPLGlu) exhibited significantly elevated burst firing accompanied by upregulation of Cav3.1 T-type calcium channel expression and function in RIH model mice. In addition, we identified a glutamatergic neuronal thalamocortical circuit in the VPL projecting to hindlimb primary somatosensory cortex glutamatergic neurons (S1HLGlu) that mediated RIH. In vivo calcium imaging and multi-tetrode recordings revealed heightened S1HLGlu neuronal activity during RIH. Moreover, preoperative suppression of Cav3.1-dependent burst firing in VPLGlu neurons or chemogenetic inhibition of VPLGlu neuronal terminals in the S1HL abolished the increased S1HLGlu neuronal excitability while alleviating RIH. Our findings suggest that remifentanil induces postoperative hyperalgesia by upregulating T-type calcium channel-dependent burst firing in VPLGlu neurons to activate S1HLGlu neurons, thus revealing an ion channel-mediated neural circuit basis for RIH that can guide analgesic development.
Keywords: Anesthesiology; Neuroscience; Pain.
Figures





Comment in
- Opioid-induced hyperalgesia: Are thalamic T-type calcium channels treatment targets? doi: 10.1172/JCI165977

