Astegolimab or Efmarodocokin Alfa in Patients With Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial
- PMID: 36519984
- PMCID: PMC9749945
- DOI: 10.1097/CCM.0000000000005716
Astegolimab or Efmarodocokin Alfa in Patients With Severe COVID-19 Pneumonia: A Randomized, Phase 2 Trial
Abstract
Objectives: Severe cases of COVID-19 pneumonia can lead to acute respiratory distress syndrome (ARDS). Release of interleukin (IL)-33, an epithelial-derived alarmin, and IL-33/ST2 pathway activation are linked with ARDS development in other viral infections. IL-22, a cytokine that modulates innate immunity through multiple regenerative and protective mechanisms in lung epithelial cells, is reduced in patients with ARDS. This study aimed to evaluate safety and efficacy of astegolimab, a human immunoglobulin G2 monoclonal antibody that selectively inhibits the IL-33 receptor, ST2, or efmarodocokin alfa, a human IL-22 fusion protein that activates IL-22 signaling, for treatment of severe COVID-19 pneumonia.
Design: Phase 2, double-blind, placebo-controlled study (COVID-astegolimab-IL).
Setting: Hospitals.
Patients: Hospitalized adults with severe COVID-19 pneumonia.
Interventions: Patients were randomized to receive IV astegolimab, efmarodocokin alfa, or placebo, plus standard of care. The primary endpoint was time to recovery, defined as time to a score of 1 or 2 on a 7-category ordinal scale by day 28.
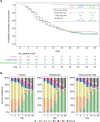
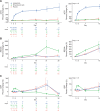
Measurements and main results: The study randomized 396 patients. Median time to recovery was 11 days (hazard ratio [HR], 1.01 d; p = 0.93) and 10 days (HR, 1.15 d; p = 0.38) for astegolimab and efmarodocokin alfa, respectively, versus 10 days for placebo. Key secondary endpoints (improved recovery, mortality, or prevention of worsening) showed no treatment benefits. No new safety signals were observed and adverse events were similar across treatment arms. Biomarkers demonstrated that both drugs were pharmacologically active.
Conclusions: Treatment with astegolimab or efmarodocokin alfa did not improve time to recovery in patients with severe COVID-19 pneumonia.
Trial registration: ClinicalTrials.gov NCT04386616.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
Dr. Waters has received funding from Genentech paid to his institution for the conduct of this trial. Dr. McKinnell has received funding from Genentech paid to his institution for the conduct of this trial; he reports receiving research funding paid to his institution from Gilead, Eli Lilly and Company, and Duke University; and he received funding from Pfizer and ThermoFisher. Dr. Martin has received compensation for service as a monitor of the COVID-astegolimab-interleukin trial. Dr. Buchman serves as a Senior Advisor to Biomedical Advanced Research and Development Authority (BARDA), U.S. Department of Health and Human Services under an agreement between his employer, Emory University, and that agency; he also serves as Editor-in-Chief of Critical Care Medicine, and Emory University similarly receives payment for that service from the Society of Critical Care Medicine. Drs. McKinnell, Martin, Buchman, Theess, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck disclosed the off-label product use of Astegolimab and Efmarodocokin Alfa. Dr. Theess is currently an employee of F. Hoffmann-La Roche and owns Roche stock. Drs. Staton and Wang are former employees of Genentech and own Roche stock. Dr. Chen is a former employee of Genentech. Drs. Martin, Theess, Yang, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Cheung, Galanter, Chen, Mohan, and Peck received funding from Genentech. Drs. Theess’s, Lekkerkerker’s, Staton’s, Rosenberger’s, Wang’s, Zhang’s, Cheung’s, Chen’s, Mohan’s, and Peck’s institutions received funding from the Department of Health and Human Services, the Office of the Assistant Secretary for Preparedness and Response, and the BARDA (OT number: HHSO100201800036C). Drs. Theess, Yang, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck are employees of Genentech, a member of the Roche group, and own Roche stock. Drs. Theess and Brooks disclosed they are employed by F. Hoffman-LaRoche. Drs. Theess, Lekkerkerker, Staton, Rosenberger, Pappu, Wang, Zhang, Brooks, Cheung, Galanter, Chen, Mohan, and Peck disclosed work for hire. Dr. Brooks disclosed that he is a Principal Computational Researcher at Genentech. Dr. Kalil has disclosed that he does not have any potential conflicts of interest.
Figures

References
-
- Wu Z, McGoogan JM: Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020; 323:1239–1242 - PubMed

