Profile of loiasis infection through clinical and laboratory diagnostics: the importance of biomarkers
- PMID: 36520072
- PMCID: PMC10153730
- DOI: 10.1093/trstmh/trac116
Profile of loiasis infection through clinical and laboratory diagnostics: the importance of biomarkers
Abstract
Background: Detection of Loa loa microfilariae in peripheral blood is insensitive given only 30% of individuals are microfilaraemic while 70% are amicrofilaraemic with a variety of clinical signs. Biomarkers may improve the diagnosis of loiasis.
Methods: A total of 545 individuals exposed to L. loa were analysed using clinical data collected through a questionnaire (requesting information on eye worm, Calabar swelling, pruritis) and detection of microfilariae, immunoglobulin G4 (IgG4), DNA and antigens using microscopy, enzyme-linked immunosorbent assay (ELISA), quantitative polymerase chain reaction (qPCR) and Western blot, respectively.
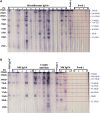
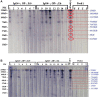
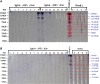
Results: The results revealed that the rates of detection of L. loa microfilariae in the blood, of DNA by qPCR, of IgG4 by ELISA and of antigen by Western blot were 4.7%, 5.5%, 15.60% and 10.09%, respectively.
Conclusions: This study showed that clinical signs based on a questionnaire are highly subjective. Therefore it is imperative to use IgG4 and DNA biomarkers as well as antigens detected by Western blot to identify individuals infected with L. loa.
Keywords: L. loa DNA; L. loa adult antigen; IgG4 response; diagnosis; loiasis profile; qPCR.
© The Author(s) 2022. Published by Oxford University Press on behalf of Royal Society of Tropical Medicine and Hygiene.
Conflict of interest statement
None declared.
Figures

Similar articles
-
IgG4 serology of loiasis in three villages in an endemic area of south-eastern Gabon.Trop Med Int Health. 1998 Apr;3(4):313-7. doi: 10.1046/j.1365-3156.1998.00224.x. Trop Med Int Health. 1998. PMID: 9623933
-
High levels of parasite-specific IgG4 in the absence of microfilaremia in Loa loa infection.Trop Med Parasitol. 1994 Sep;45(3):246-8. Trop Med Parasitol. 1994. PMID: 7899797
-
[Comparative analysis of 2 diagnostic methods of human loiasis: IgG4 serology and nested PCR].Bull Soc Pathol Exot. 1999 Jul;92(3):167-70. Bull Soc Pathol Exot. 1999. PMID: 10472442 French.
-
Imported Loa loa filariasis: three cases and a review of cases reported in non-endemic countries in the past 25 years.Int J Infect Dis. 2012 Sep;16(9):e649-62. doi: 10.1016/j.ijid.2012.05.1023. Epub 2012 Jul 10. Int J Infect Dis. 2012. PMID: 22784545 Review.
-
Loa loa infection detection using biomarkers: current perspectives.Res Rep Trop Med. 2018 Apr 3;9:43-48. doi: 10.2147/RRTM.S132380. eCollection 2018. Res Rep Trop Med. 2018. PMID: 30050354 Free PMC article. Review.
Cited by
-
Analysis of diagnostic test outcomes in a large loiasis cohort from an endemic region: Serological tests are often false negative in hyper-microfilaremic infections.PLoS Negl Trop Dis. 2024 Mar 14;18(3):e0012054. doi: 10.1371/journal.pntd.0012054. eCollection 2024 Mar. PLoS Negl Trop Dis. 2024. PMID: 38484012 Free PMC article.
-
Diagnosis, management and prevention of loiasis: guideline of the German Society for Tropical Medicine, Travel Medicine, and Global Health (DTG).Infection. 2025 Jun;53(3):851-872. doi: 10.1007/s15010-024-02443-2. Epub 2025 May 21. Infection. 2025. PMID: 40397272 Free PMC article.
References
-
- Navarro M, Camprubí D, Requena-Méndez Aet al. . Safety of high-dose ivermectin: a systematic review and meta-analysis. J Antimicrob Chemother. 2020;75(4):827–34. - PubMed
-
- Pion S, Chesnais C. Loiasis. In: Marcondes C (editor). Arthropod Borne Diseases. Cham: Springer; 2017. 10.1007/978-3-319-13884-8_27. - DOI
-
- Chesnais CB, Takougang I, Paguélé Met al. . Excess mortality associated with loiasis: a retrospective population-based cohort study. Lancet Infect Dis. 2017;17(1):108–16. - PubMed
-
- Antinori S, Schifanella L, Million Met al. . Imported Loa loa filariasis: three cases and a review of cases reported in non-endemic countries in the past 25 years. Int J Infect Dis. 2012;16(9):e649–62. - PubMed
-
- Dupont A, Zue-N'dong J, Pinder M.. Common occurrence of amicrofilaraemic Loa loa filariasis within the endemic region. Trans R Soc Trop Med Hyg. 1988;82(5):730. - PubMed

