Targeting Nitric Oxide: Say NO to Metastasis
- PMID: 36520504
- PMCID: PMC10183809
- DOI: 10.1158/1078-0432.CCR-22-2791
Targeting Nitric Oxide: Say NO to Metastasis
Abstract
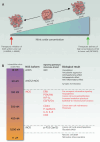
Utilizing targeted therapies capable of reducing cancer metastasis, targeting chemoresistant and self-renewing cancer stem cells, and augmenting the efficacy of systemic chemo/radiotherapies is vital to minimize cancer-associated mortality. Targeting nitric oxide synthase (NOS), a protein within the tumor microenvironment, has gained interest as a promising therapeutic strategy to reduce metastatic capacity and augment the efficacy of chemo/radiotherapies in various solid malignancies. Our review highlights the influence of nitric oxide (NO) in tumor progression and cancer metastasis, as well as promising preclinical studies that evaluated NOS inhibitors as anticancer therapies. Lastly, we highlight the prospects and outstanding challenges of using NOS inhibitors in the clinical setting.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Dong Z, Staroselsky AH, Qi X, Xie K, Fidler IJ. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res 1994;54:789–93. - PubMed
-
- Xie K, Dong Z, Fidler IJ. Activation of nitric oxide synthase gene for inhibition of cancer metastasis. J Leukoc Biol 1996;59:797–803. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

