Microglial pattern recognition via IL-33 promotes synaptic refinement in developing corticothalamic circuits in mice
- PMID: 36520518
- PMCID: PMC9757845
- DOI: 10.1084/jem.20220605
Microglial pattern recognition via IL-33 promotes synaptic refinement in developing corticothalamic circuits in mice
Abstract
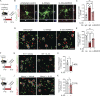
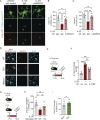
Microglia are critical regulators of brain development that engulf synaptic proteins during postnatal synapse remodeling. However, the mechanisms through which microglia sense the brain environment are not well defined. Here, we characterized the regulatory program downstream of interleukin-33 (IL-33), a cytokine that promotes microglial synapse remodeling. Exposing the developing brain to a supraphysiological dose of IL-33 altered the microglial enhancer landscape and increased binding of stimulus-dependent transcription factors including AP-1/FOS. This induced a gene expression program enriched for the expression of pattern recognition receptors, including the scavenger receptor MARCO. CNS-specific deletion of IL-33 led to increased excitatory/inhibitory synaptic balance, spontaneous absence-like epileptiform activity in juvenile mice, and increased seizure susceptibility in response to chemoconvulsants. We found that MARCO promoted synapse engulfment, and Marco-deficient animals had excess thalamic excitatory synapses and increased seizure susceptibility. Taken together, these data define coordinated epigenetic and functional changes in microglia and uncover pattern recognition receptors as potential regulators of postnatal synaptic refinement.
© 2022 Han et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures