A Role for Early-Phase Transmission in the Enzootic Maintenance of Plague
- PMID: 36520713
- PMCID: PMC9754260
- DOI: 10.1371/journal.ppat.1010996
A Role for Early-Phase Transmission in the Enzootic Maintenance of Plague
Abstract
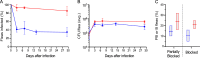
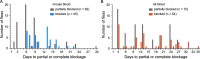
Yersinia pestis, the bacterial agent of plague, is enzootic in many parts of the world within wild rodent populations and is transmitted by different flea vectors. The ecology of plague is complex, with rodent hosts exhibiting varying susceptibilities to overt disease and their fleas exhibiting varying levels of vector competence. A long-standing question in plague ecology concerns the conditions that lead to occasional epizootics among susceptible rodents. Many factors are involved, but a major one is the transmission efficiency of the flea vector. In this study, using Oropsylla montana (a ground squirrel flea that is a major plague vector in the western United States), we comparatively quantified the efficiency of the two basic modes of flea-borne transmission. Transmission efficiency by the early-phase mechanism was strongly affected by the host blood source. Subsequent biofilm-dependent transmission by blocked fleas was less influenced by host blood and was more efficient. Mathematical modeling predicted that early-phase transmission could drive an epizootic only among highly susceptible rodents with certain blood characteristics, but that transmission by blocked O. montana could do so in more resistant hosts irrespective of their blood characteristics. The models further suggested that for most wild rodents, exposure to sublethal doses of Y. pestis transmitted during the early phase may restrain rapid epizootic spread by increasing the number of immune, resistant individuals in the population.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Gage KL, Kosoy M. Recent trends in plague ecology. In: Roelle J, Miller B, Godbey J, Biggins D, editors. Recovery of the black-footed ferret, progress and continuing challenges. Fort. Collins, CO: U. S. Geological Survey; 2006. p. 213–31.
-
- Wimsatt J, Biggins DE. A review of plague persistence with special emphasis on fleas. J Vector Borne Dis. 2009;46:85–99. - PubMed
-
- Dubyanskiy VM, Yeszhanov AB. Ecology of Yersinia pestis and the epidemiology of plague. Adv Exp Med Biol. 2016;918:101–70. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

