Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: randomised controlled trial
- PMID: 36520715
- PMCID: PMC9682494
- DOI: 10.1136/bmj-2022-072623
Rivaroxaban treatment for six weeks versus three months in patients with symptomatic isolated distal deep vein thrombosis: randomised controlled trial
Abstract
Objective: To compare two different treatment durations of rivaroxaban in patients with symptomatic isolated distal deep vein thrombosis (DVT).
Design: Randomised, double blind, placebo controlled clinical trial.
Setting: 28 outpatient clinics specialising in venous thromboembolism.
Participants: 402 adults (≥18 years) with symptomatic isolated distal DVT.
Interventions: After receiving standard dose rivaroxaban for six weeks, participants were randomly assigned to receive rivaroxaban 20 mg or placebo once daily for an additional six weeks. Follow-up was for 24 months from study inclusion.
Main outcomes measures: The primary efficacy outcome was recurrent venous thromboembolism during follow-up after randomisation, defined as the composite of progression of isolated distal DVT, recurrent isolated distal DVT, proximal DVT, symptomatic pulmonary embolism, or fatal pulmonary embolism. The primary safety outcome was major bleeding after randomisation until two days from the last dose of rivaroxaban or placebo. An independent committee adjudicated the outcomes.
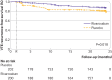
Results: 200 adults were randomised to receive additional rivaroxaban treatment and 202 to receive placebo. Isolated distal DVT was unprovoked in 81 (40%) and 86 (43%) patients, respectively. The primary efficacy outcome occurred in 23 (11%) patients in the rivaroxaban arm and 39 (19%) in the placebo arm (relative risk 0.59, 95% confidence interval 0.36 to 0.95; P=0.03, number needed to treat 13, 95% confidence interval 7 to 126). Recurrent isolated distal DVT occurred in 16 (8%) patients in the rivaroxaban arm and 31 (15%) in the placebo arm (P=0.02). Proximal DVT or pulmonary embolism occurred in seven (3%) patients in the rivaroxaban arm and eight (4%) in the placebo arm (P=0.80). No major bleeding events occurred.
Conclusions: Rivaroxaban administered for six additional weeks in patients with isolated distal DVT who had an uneventful six week treatment course reduces the risk of recurrent venous thromboembolism, mainly recurrent isolated distal DVT, over a two year follow-up without increasing the risk of haemorrhage.
Trial registration: EudraCT 2016-000958-36; ClinicalTrials.gov NCT02722447.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure for at www.icmje.org/disclosure-of-interest/ and declare: support from Bayer Italy. WA has received payment or honorariums for lectures from Aspen, Bayer, Bristol Myers Squibb, Leo Pharma, Norgine, Pfizer, Sanofi, and Werfen, and has participated on advisory boards for Bayer, Leo Pharma, Norgine, Sanofi, and Viatris; GP has received consulting fees from Alfa Sigma. All other authors have no financial relationships or other relationships or activities to disclose.
Figures


Comment in
-
In symptomatic isolated DVT, 12 wk vs. 6 wk of rivaroxaban reduced recurrent VTE at 24 mo.Ann Intern Med. 2023 Apr;176(4):JC42. doi: 10.7326/J23-0014. Epub 2023 Apr 4. Ann Intern Med. 2023. PMID: 37011394
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
