Multidimensional control of therapeutic human cell function with synthetic gene circuits
- PMID: 36520914
- PMCID: PMC10054295
- DOI: 10.1126/science.ade0156
Multidimensional control of therapeutic human cell function with synthetic gene circuits
Abstract
Synthetic gene circuits that precisely control human cell function could expand the capabilities of gene- and cell-based therapies. However, platforms for developing circuits in primary human cells that drive robust functional changes in vivo and have compositions suitable for clinical use are lacking. Here, we developed synthetic zinc finger transcription regulators (synZiFTRs), which are compact and based largely on human-derived proteins. As a proof of principle, we engineered gene switches and circuits that allow precise, user-defined control over therapeutically relevant genes in primary T cells using orthogonal, US Food and Drug Administration-approved small-molecule inducers. Our circuits can instruct T cells to sequentially activate multiple cellular programs such as proliferation and antitumor activity to drive synergistic therapeutic responses. This platform should accelerate the development and clinical translation of synthetic gene circuits in diverse human cell types and contexts.
Conflict of interest statement
Figures

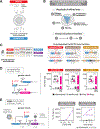

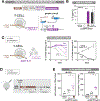
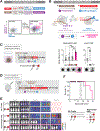
Comment in
-
Control of CAR-T cell activity in space and time: the next level of anti-tumor action.Signal Transduct Target Ther. 2023 Apr 10;8(1):150. doi: 10.1038/s41392-023-01424-5. Signal Transduct Target Ther. 2023. PMID: 37037800 Free PMC article. No abstract available.
References
-
- Xie M, Fussenegger M, Designing cell function: assembly of synthetic gene circuits for cell biology applications. Nat Rev Mol Cell Biol 19, 507–525 (2018). - PubMed
-
- Hay KA, Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol 183, 364–374 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

