The enteric nervous system
- PMID: 36521049
- PMCID: PMC9970663
- DOI: 10.1152/physrev.00018.2022
The enteric nervous system
Abstract
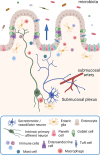
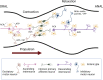
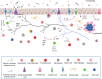
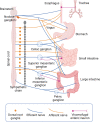
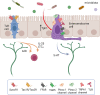
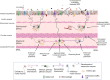
Of all the organ systems in the body, the gastrointestinal tract is the most complicated in terms of the numbers of structures involved, each with different functions, and the numbers and types of signaling molecules utilized. The digestion of food and absorption of nutrients, electrolytes, and water occurs in a hostile luminal environment that contains a large and diverse microbiota. At the core of regulatory control of the digestive and defensive functions of the gastrointestinal tract is the enteric nervous system (ENS), a complex system of neurons and glia in the gut wall. In this review, we discuss 1) the intrinsic neural control of gut functions involved in digestion and 2) how the ENS interacts with the immune system, gut microbiota, and epithelium to maintain mucosal defense and barrier function. We highlight developments that have revolutionized our understanding of the physiology and pathophysiology of enteric neural control. These include a new understanding of the molecular architecture of the ENS, the organization and function of enteric motor circuits, and the roles of enteric glia. We explore the transduction of luminal stimuli by enteroendocrine cells, the regulation of intestinal barrier function by enteric neurons and glia, local immune control by the ENS, and the role of the gut microbiota in regulating the structure and function of the ENS. Multifunctional enteric neurons work together with enteric glial cells, macrophages, interstitial cells, and enteroendocrine cells integrating an array of signals to initiate outputs that are precisely regulated in space and time to control digestion and intestinal homeostasis.
Keywords: enteric glia; enteric nervous system; interstitial cells of Cajal; myenteric plexus; vagus nerve.
Conflict of interest statement
K.A.S. has provided scientific advice and assistance to Arena Pharmaceuticals and GW Pharmaceuticals, has served on a speaker bureau for Abbvie, and has received research support from Takeda Pharmaceuticals and Abalone, Inc. G.M.M. has received research support from Takeda Pharmaceuticals and is on the Scientific Advisory Board of Dignify Therapeutics.
Figures





References
-
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone, 1987.
-
- Furness JB. The Enteric Nervous System. Oxford, UK: Blackwell, 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

