Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma
- PMID: 36521103
- PMCID: PMC9995096
- DOI: 10.1200/JCO.22.00996
Phase IIa Study of SurVaxM Plus Adjuvant Temozolomide for Newly Diagnosed Glioblastoma
Abstract
Purpose: Despite intensive treatment with surgery, radiation therapy, temozolomide (TMZ) chemotherapy, and tumor-treating fields, mortality of newly diagnosed glioblastoma (nGBM) remains very high. SurVaxM is a peptide vaccine conjugate that has been shown to activate the immune system against its target molecule survivin, which is highly expressed by glioblastoma cells. We conducted a phase IIa, open-label, multicenter trial evaluating the safety, immunologic effects, and survival of patients with nGBM receiving SurVaxM plus adjuvant TMZ following surgery and chemoradiation (ClinicalTrials.gov identifier: NCT02455557).
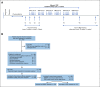
Methods: Sixty-four patients with resected nGBM were enrolled including 38 men and 26 women, in the age range of 20-82 years. Following craniotomy and fractionated radiation therapy with concurrent TMZ, patients received four doses of SurVaxM (500 μg once every 2 weeks) in Montanide ISA-51 plus sargramostim (granulocyte macrophage colony-stimulating factor) subcutaneously. Patients subsequently received adjuvant TMZ and maintenance SurVaxM concurrently until progression. Progression-free survival (PFS) and overall survival (OS) were reported. Immunologic responses to SurVaxM were assessed.
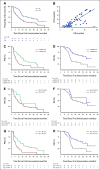
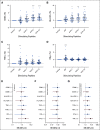
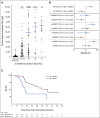
Results: SurVaxM plus TMZ was well tolerated with no serious adverse events attributable to SurVaxM. Of the 63 patients who were evaluable for outcome, 60 (95.2%) remained progression-free 6 months after diagnosis (prespecified primary end point). Median PFS was 11.4 months and median OS was 25.9 months measured from first dose of SurVaxM. SurVaxM produced survivin-specific CD8+ T cells and antibody/immunoglobulin G titers. Apparent clinical benefit of SurVaxM was observed in both methylated and unmethylated patients.
Conclusion: SurVaxM appeared to be safe and well tolerated. The combination represents a promising therapy for nGBM. For patients with nGBM treated in this manner, PFS may be an acceptable surrogate for OS. A large randomized clinical trial of SurVaxM for nGBM is in progress.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures


References
-
- Stupp R, Mason WP, van den Bent MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005 - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. : MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997-1003, 2005 - PubMed
-
- Ambrosini G, Adida C, Altieri DC: A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917-921, 1997 - PubMed
-
- Chakravarti A, Noll E, Black PM, et al. : Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol 20:1063-1068, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

