Anxiolytic reversal of classically conditioned / chronic stress-induced gene expression and learning in the Stress Alternatives Model
- PMID: 36521572
- PMCID: PMC9872777
- DOI: 10.1016/j.bbr.2022.114258
Anxiolytic reversal of classically conditioned / chronic stress-induced gene expression and learning in the Stress Alternatives Model
Abstract
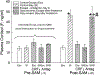
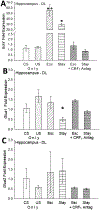
Social decision-making is critically influenced by neurocircuitries that regulate stress responsiveness. Adaptive choices, therefore, are altered by stress-related neuromodulatory peptide systems, such as corticotropin releasing factor (CRF). Experimental designs that take advantage of ecologically salient fear-inducing stimuli allow for revelation of neural mechanisms that regulate the balance between pro- and anti-stress responsiveness. To accomplish this, we developed a social stress and conditioning protocol, the Stress Alternatives Model (SAM), that utilizes a simple dichotomous choice, and produces distinctive behavioral phenotypes (Escape or Stay). The experiments involve repeated social aggression, a potent unconditioned stimulus (US), from a novel larger conspecific (a 3X larger Rainbow trout). Prior to the social interaction, the smaller test fish is presented with an auditory conditioning stimulus (water off = CS). During the social aggression, an escape route is available, but is only large enough for the smaller test animal. Surprisingly, although the new aggressor provides vigorous attacks each day, only 50% of the test fish choose Escape. Stay fish, treated with the CRF1 antagonist antalarmin, a potent anxiolytic drug, on day 4, promotes Escape behavior for the last 4 days of the SAM protocol. The results suggest that the decision to Escape, required a reduction in stress reactivity. The Stay fish that chose Escape following anxiolytic treatment, learned how to use the escape route prior to stress reduction, as the Escape latency in these fish was significantly faster than first time escapers. In Escape fish, the use of the escape route is learned over several days, reducing the Escape latency over time in the SAM. Fear conditioning (water off + aggression) resulted in elevated hippocampal (DL) Bdnf mRNA levels, with coincident reduction in the AMPA receptor subunit Glua1 expression, a result that is reversed following a one-time treatment (during SAM aggression on day 4) with the anxiolytic CRF1 receptor antagonist antalarmin.
Keywords: Anxiety; Corticotropin-releasing factor; Depression; Fear conditioning; PTSD; SAM; Social aggression.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest Authors report no conflict of interest.
Figures

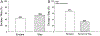

References
-
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S, Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning, Brain research 796(1–2) (1998) 132–42. - PubMed
-
- Blanchard RJ, Nikulina JN, Sakai RR, McKittrick C, McEwen B, Blanchard DC, Behavioral and endocrine change following chronic predatory stress, Physiology & behavior 63(4) (1998) 561–9. - PubMed
-
- Wallace KJ, Rosen JB, Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces, Behavioral neuroscience 114(5) (2000) 912–922. - PubMed
-
- Endres T, Fendt M, Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline, Behavioral neuroscience 121(3) (2007) 594–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

