Ion-Induced Transient Potential Fluctuations Facilitate Pore Formation and Cation Transport through Lipid Membranes
- PMID: 36521841
- PMCID: PMC9801421
- DOI: 10.1021/jacs.2c08543
Ion-Induced Transient Potential Fluctuations Facilitate Pore Formation and Cation Transport through Lipid Membranes
Abstract
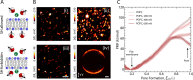
Unassisted ion transport through lipid membranes plays a crucial role in many cell functions without which life would not be possible, yet the precise mechanism behind the process remains unknown due to its molecular complexity. Here, we demonstrate a direct link between membrane potential fluctuations and divalent ion transport. High-throughput wide-field non-resonant second harmonic (SH) microscopy of membrane water shows that membrane potential fluctuations are universally found in lipid bilayer systems. Molecular dynamics simulations reveal that such variations in membrane potential reduce the free energy cost of transient pore formation and increase the ion flux across an open pore. These transient pores can act as conduits for ion transport, which we SH image for a series of divalent cations (Cu2+, Ca2+, Ba2+, Mg2+) passing through giant unilamellar vesicle (GUV) membranes. Combining the experimental and computational results, we show that permeation through pores formed via an ion-induced electrostatic field is a viable mechanism for unassisted ion transport.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Bordi F.; Cametti C.; Motta A. Ion Permeation Across Model Lipid Membranes: A Kinetic Approach. J. Phys. Chem. B 2000, 104, 5318–5323. 10.1021/jp000005i. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

