Effectiveness, usability and acceptability of a smart inhaler programme in patients with asthma: protocol of the multicentre, pragmatic, open-label, cluster randomised controlled ACCEPTANCE trial
- PMID: 36522130
- PMCID: PMC9756226
- DOI: 10.1136/bmjresp-2022-001400
Effectiveness, usability and acceptability of a smart inhaler programme in patients with asthma: protocol of the multicentre, pragmatic, open-label, cluster randomised controlled ACCEPTANCE trial
Abstract
Introduction: Suboptimal asthma control is associated with incorrect inhaler use and poor medication adherence, which could lead to unfavourable clinical and economic outcomes. Smart inhaler programmes using electronic monitoring devices (EMDs) could support self-management and increase medication adherence and asthma control. However, evidence on long-term benefits and acceptability is scarce. This study aims to investigate the effectiveness of a smart inhaler asthma self-management programme on medication adherence and clinical outcomes in adults with uncontrolled asthma, to evaluate its acceptability and to identify subgroups who would benefit most based on patient characteristics.
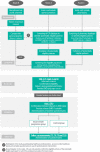
Methods and analysis: This open-label cluster randomised controlled trial of 12 months will be conducted in primary care in the Netherlands. General practices will be randomly assigned to either intervention or control group. We aim to include 242 patients. The intervention consists of (1) an EMD attached to the patient's inhaler that measures medication use; (2) a smartphone application to set medication reminders, receive motivational messages and track asthma symptoms; and (3) a portal for healthcare professionals to view data on medication use. The control group is passively monitored by the EMD but cannot view their inhaler data or receive feedback. Eligible patients are adults with suboptimal controlled asthma (Asthma Control Questionnaire score ≥0.75) with evidence of non-adherence established by the EMD during a 6-week run-in period. Primary outcome is the difference in mean medication adherence between intervention and control group. Secondary outcomes include asthma control, asthma-related quality of life, exacerbations, acceptance, cost-effectiveness and whether the effect of the intervention on medication adherence and asthma control is modified by patient characteristics (eg, self-efficacy, medication beliefs and eHealth literacy).Trial registration numberNL7854.
Keywords: Asthma; Asthma in primary care; Inhaler devices.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JWK reports grants, personal fees and non-financial support outside the submitted work from AstraZeneca; grants, personal fees and non-financial support from Boehringer Ingelheim and GSK; grants and personal fees from Chiesi Pharmaceuticals and TEVA; grants from Mundi Pharma; and personal fees from MSD and COVIS Pharma. JWK also holds 72.5% of shares in the General Practitioners Research Institute. JFMvB received grants and/or consultancy fees from AstraZeneca, Chiesi, European Commission COST (COST Action 19132), GSK, Novartis, Teva and Trudell Medical, outside the submitted work and all paid to his institution. BFdB was employed by General Practitioners Research Institute (GPRI) at the time of the study. In the past three years (2019–2021), GPRI conducted investigator-initiated and sponsor-initiated research funded by non-commercial organisations, academic institutes and pharmaceutical companies (including AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Mundipharma, Novartis and Teva).
Figures
References
-
- Haselkorn T, Fish JE, Zeiger RS, et al. . Consistently very poorly controlled asthma, as defined by the impairment domain of the expert panel report 3 guidelines, increases risk for future severe asthma exacerbations in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study. J Allergy Clin Immunol 2009;124:895–902. 10.1016/j.jaci.2009.07.035 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical