Convergent evolution and horizontal gene transfer in Arctic Ocean microalgae
- PMID: 36522135
- PMCID: PMC9756366
- DOI: 10.26508/lsa.202201833
Convergent evolution and horizontal gene transfer in Arctic Ocean microalgae
Abstract
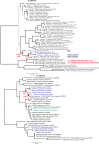
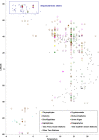
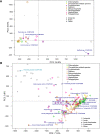
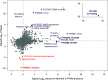
Microbial communities in the world ocean are affected strongly by oceanic circulation, creating characteristic marine biomes. The high connectivity of most of the ocean makes it difficult to disentangle selective retention of colonizing genotypes (with traits suited to biome specific conditions) from evolutionary selection, which would act on founder genotypes over time. The Arctic Ocean is exceptional with limited exchange with other oceans and ice covered since the last ice age. To test whether Arctic microalgal lineages evolved apart from algae in the global ocean, we sequenced four lineages of microalgae isolated from Arctic waters and sea ice. Here we show convergent evolution and highlight geographically limited HGT as an ecological adaptive force in the form of PFAM complements and horizontal acquisition of key adaptive genes. Notably, ice-binding proteins were acquired and horizontally transferred among Arctic strains. A comparison with Tara Oceans metagenomes and metatranscriptomes confirmed mostly Arctic distributions of these IBPs. The phylogeny of Arctic-specific genes indicated that these events were independent of bacterial-sourced HGTs in Antarctic Southern Ocean microalgae.
© 2022 Dorrell et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures











References
-
- Longhurst A (2006). Ecological Geography of the Sea, pp 560. Cambridge: Academic Press.
-
- Carmack EC (2007) The alpha/beta ocean distinction: A perspective on freshwater fluxes, convection, nutrients and productivity in high-latitude seas. Deep Sea Res Part II: Topical Stud Oceanogr 54: 2578–2598. 10.1016/j.dsr2.2007.08.018 - DOI
-
- Beszczynska-Moller A, Woodgate RA, Lee C, Melling H, Karcher M (2011) A synthesis of exchanges through the main oceanic gateways to the Arctic Ocean. Oceanography 24: 82–99. 10.5670/oceanog.2011.59 - DOI
